2 Minute Medicine Rewind July 29, 2019
Roxadustat for anemia in patients with kidney disease not receiving dialysis
Chronic kidney disease (CKD)-related anemia is associated with significant morbidity, and remains undertreated worldwide. Adequate treatment of CKD-related anemia is associated with reduced transfusion rates and improved clinical outcomes. Roxadustat is a hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor that stabilizes HIF-alpha subunits, resulting in increased transcriptional activity and functional activation of target genes encoding erythropoietin, erythropoein receptor, heme biosynthesis enzymes, iron absorption and transport proteins. In this phase III trial of Chinese patients with CKD not undergoing dialysis, 154 patients were randomized to receive roxadustat three times a week for 8 weeks or placebo to evaluate the efficacy of roxadustat in the treatment of anemia in this patient population. This was followed by an 18-week open-label phase where all patients received roxadustat, and parenteral iron was held. The primary outcome of interest was mean change from baseline in hemoglobin level, averaged between weeks 7 and 9. Researchers found that the mean change from baseline hemoglobin level was 1.9 g/dL (SD 1.22 g/dL) with roxadustat versus 0.4 g/dL (SD 0.8 g/dL) in the control group (difference 2.2 g/dL, 95% CI 1.9 g/dL to 2.6 g/dL, p<0.001). A hemoglobin response of at least 1.0 g/dL increase from baseline by week 9 was observed in 85 (84%) patients receiving roxadustat and 0 (0%) patients in the control group (difference 84%, 95% CI 75% to 91%). The roxadustat group also demonstrated significantly greater mean reductions from baseline in hepcidin and total cholesterol levels, and more frequent episodes of hyperkalemia and metabolic acidosis. The efficacy of roxadustat was maintained during the 18-week open-label period in terms of improvement and maintenance of hemoglobin. The findings of this study therefore show that roxadustat may be effective in CKD patients not on dialysis in terms of correcting and maintaining hemoglobin levels in the setting of anemia.
Overall survival with ribociclib plus endocrine therapy in breast cancer
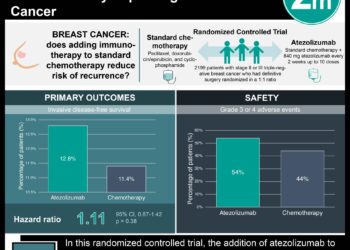
Hormone therapy is the standard of care for premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor2 (HER2)-negative advanced breast cancer. Ribociclib is an orally administered CDK4/6 inhibitor. Multiple trials have shown that combined ribociclib and endocrine therapy result in significantly improved progression-free survival compared to endocrine therapy alone in this setting; however,no previous trials have shown a definitive improvement in overall survival. In this second interim analysis from Mammary Oncology Assessment of LEE011’s [Ribociclib’s] Efficacy and Safety-7 (MONALEESA-7), an international, randomized, double-blind placebo-controlled phase III trial, researchers compared ribociclib versus placebo in addition to endocrine therapy with goserelin and either a nonsteroidal aromatase inhibitor or tamoxifen, in women age 18 to 59 years with histologically or cytologically confirmed hormone receptor-positive, HER2-negative advanced breast cancer. Overall survival was the main outcome for this secondary analysis. Of 672 patients included in the intention-to-treat population, 83 (24.8%) and 109 (32.3%) deaths occurred in the ribociclib and placebo groups, respectively. The addition of ribociclib resulted in significantly longer overall survival (70.2% vs. 46.0% alive at 42 months; HR 0.71, 95% CI 0.54 to 0.95, p=0.00973). This benefit was also seen in the subgroup of patients receiving aromatase inhibitors as hormonal therapy. This secondary analysis from the MONALEESA-7 trial therefore shows significantly longer overall survival associated with the addition of a CDK4/6 inhibitor to baseline endocrine therapy for patients with advanced hormone receptor-positive, HER2-negative breast cancer.
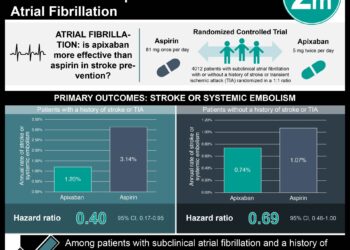
Aspirin (ASA) continues to be used for the primary prevention of cardiovascular disease (CVD) in patients at increased risk, despite evidence consistently demonstrating little benefit in cardiovascular event reduction and increased bleeding risk. Accordingly, the American Heart Association and American College of Cardiology (AHA/ACC) released new guidelines recommending against routine aspirin use in patients age 70 years and older and those at increased bleeding risk. The objective of this study was to characterize ASA use for primary prevention, in light of new guideline recommendations. The National Health Interview Survey (NHIS), a nationally-representative in-person household survey of American adult health and disability, asked participants age 40 years or older whether they were advised by a health care professional to take low-dose ASA to prevent or control heart disease, whether they followed such advice, and whether they independently took low-dose ASA (without recommendation). Participants with self-reported CVD history were excluded. Of 14,328 adults (mean age 57.5 years) without CVD, nearly 30 million participants (23.4%) reported daily ASA use. When stratified by age, 44.6% of those age 70 to 79 years and 46.2% of those over 80 years endorsed the same. Of those endorsing daily ASA use, 22.8% did so without a physician’s recommendation. Given recent recommendations against daily ASA use in those age 70 years or older, these findings suggest current practices are not in keeping with new guidelines for primary prevention. These findings also indicate that almost a quarter of patients using ASA daily for primary prevention may be doing so without their physician’s advice, and possibly without their knowledge. This underlines an increased need for health care providers to identify ASA use and provide appropriate counseling regarding risks and benefits.
A multicenter trial of vena cava filters in severely injured patients
Venous thromboembolism (VTE) is common following major trauma. While delay in thromboprophylaxis initiation by more than 1 to 3 days is associated with a significantly increased risk of VTE and mortality, prophylaxis is also associated with an increased risk of progressive hematoma enlargement in the setting of traumatic brain injury. Retrievable inferior vena cava (IVC) filters have been introduced to address this issue as a primary means of pulmonary embolism (PE) prophylaxis, though there is a lack of high-quality evidence to support their use in this setting. In this multi-centered randomized controlled trial, 240 severely injured patients were randomized to retrievable IVC filter insertion or no filter within 72 hours after admission for trauma, where prophylactic anticoagulation is contraindicated, to determine whether this intervention results in decreased incidence of PE. Researchers found that early IVC filter placement did not result in a significantly lower incidence of symptomatic PE or mortality (13.9% in IVC filter group vs. 14.4% in control group; HR 0.99, 95% CI 0.51 to 1.94, p=0.98). Of 46 and 34 patients in the IVC filter and control groups, respectively, not receiving prophylactic anticoagulation within 7 days post-injury, PE developed in 0% of the IVC filter arm and 14.7% of the control arm (RR 0.00, 95% CI 0.00 to 0.55). Entrapped filter-associated thrombus was found in 6 patients. This study therefore shows that early IVC filter placement for PE prophylaxis was not associated with a lower incidence of symptomatic PE or all-cause mortality at 90 days in patients admitted for major traumatic injury
Erdafitinib in locally advanced or metastatic urothelial carcinoma
Second-line single-agent chemotherapies or immune checkpoint inhibitors have provided suboptimal response rates and survival benefits in patients with locally advanced and unresectable or metastatic urothelial carcinoma. Certain types of urothelial carcinomas, such as the luminal I subtype, have higher percentages of mutations in the genes encoding fibroblast growth factor receptor (FGFR), associated with immune response; such alterations are relatively common in urothelial carcinoma in general, decreasing sensitivity to immune interventions. Erdafitinib is a potent tyrosine kinase inhibitor of FGFR1-4, and has shown anti-tumor activity in preclinical models and a phase I study involving patients with FGFR mutations. The objective of this uncontrolled, multicenter open-label phase II study was to assess the response of erdafitinib in patients with locally advanced and unresectable or metastatic urothelial carcinoma with FGFR alterations. Ninety-nine patients with a history of disease progression during or after at least one course of first-line chemotherapy or within 12 months after neoadjuvant or adjuvant chemotherapy were enrolled. They were initially randomly assigned to receive 28-day cycles of either intermittent or continuous regimens. Based on a planned interim analysis, further enrolment to the intermittent regimen group was stopped. Researchers found that 40% (95% CI 31% to 50%) had a confirmed response (3% complete, 37% partial); response rates were similar regardless of previous chemotherapy, number of previous treatment courses, or baseline characteristics. Of those who underwent previous immunotherapy, 59% of patients had a confirmed response. Median progression-free survival was 5.5 months, and overall survival duration was 13.8 months. Treatment-related adverse events of grade III or higher were noted in 46% of patients, with 13% of patients relatedly discontinuing therapy. No treatment-related deaths were reported. This study therefore shows that among patients with locally advanced and unresectable or metastatic urothelial carcinomas with FGFR alterations, objective partial or complete response was achieved in more than 40% of previously treated patients with erdaftinib therapy, though therapy was associated with significant toxicity and associated medication discontinuation.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.