2 Minute Medicine Rewind June 30 – July 7, 2014
In this section, we highlight the key high-impact studies, updates, and analyses published in medicine during the past week.
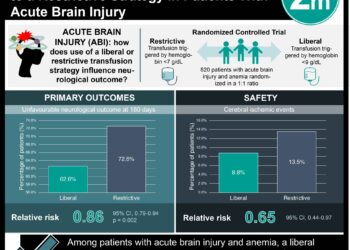
Patients with traumatic brain injury (TBI) commonly develop anemia, which can cause secondary injury and worsen neurological outcomes. Erythropoietin has anti-inflammatory, antiapoptotic, and vascular actions shown to improve outcomes after injury in experimental models and may not carry to the increased risk of infection and multiorgan failure associated with pRBC transfusions. In this randomized clinical trial, 200 patients with closed head injury and unable to follow commands were randomized to either IV erythropoietin or saline (dosed daily for 3 days and then weekly for 2 more weeks in the first dosing regimen and dosed once daily for 1 day in the second regimen). Half of the patients were assigned to a hemoglobin transfusion threshold of 7 g/dL while the other half was assigned to one of 10 g/dL (thresholds were maintained with pRBC transfusions). Compared with placebo (favorable outcome rate: 38.2%; 95% CI, 28.1% to 49.1%), neither erythropoietin groups showed significant improvement (first dosing regimen: 48.6%; 95% CI, 31.4% to 66.0%, P = .13; second dosing regimen: 29.8%; 95% CI, 18.4% to 43.4%, P < .001). In addition, thromboembolic events had a higher incidence for the transfusion threshold of 10 g/dL (21.8% vs 8.1% for the threshold of 7 g/dL, odds ratio, 0.32 [95% CI, 0.12 to 0.79], P = .009). Thus, in patients with TBI, the administration of erythropoietin did not result in improved outcomes.
A Randomized Trial of Epidural Glucocorticoid Injections for Spinal Stenosis
Epidural glucocorticoid injections are an increasingly common treatment for spinal stenosis (more than 2.2 million lumbar epidural glucocorticoid injections are performed each year in the Medicare population); however, there remains a lack of data from rigorous randomized, controlled trials evaluating their effectiveness and safety. In this double-blind, multisite trial, 400 patients with lumbar central spinal stenosis and moderate-to-severe leg pain were randomized to receive epidural injections of glucocorticoids plus lidocaine or lidocaine alone. Six weeks post-treatment, the Roland–Morris Disability Questionnaire score, used to assess physical disability, was not significantly different between the two groups (adjusted difference in the average treatment effect, −1.0 points; 95% confidence interval [CI], −2.1 to 0.1; P=0.07). Similarly, the intensity of leg pain on a scale of 1 to 10 was not significantly different (adjusted difference in the average treatment effect, −0.2 points; 95% CI, −0.8 to 0.4; P=0.48). Thus, this study shows no advantage to administering a glucocorticoid–lidocaine injection over a lidocaine-alone injection in patients with spinal stenosis.
Risk of Pediatric Celiac Disease According to HLA Haplotype and Country
The etiology of celiac disease is multifactorial and involves a genetic component; the presence of HLA haplotype DR3–DQ2 or DR4–DQ8 has been associated with an increased risk of developing the disease. In this study, 6403 American, Finnish, German and Swedish children with HLA haplotype DR3–DQ2 or DR4–DQ8 were followed prospectively from birth. Twelve percent of children developed celiac disease autoimmunity, defined as the presence of tTG antibodies on two consecutive tests at least 3 months apart. Amongst children with a single DR3–DQ2 haplotype, the risks of celiac disease autoimmunity and celiac disease by the age of 5 years were 11% and 3%, respectively, while it was 26% and 11%, respectively, amongst those with two copies (DR3–DQ2 homozygosity). Sweden residence was independently associated with an increased risk of celiac disease autoimmunity (hazard ratio, 1.90; 95% CI, 1.61 to 2.25). This study reinforces the genetic (HLA haplotype DR3–DQ2 homozygotes were at highest risk) as well as environmental (the higher riskassociated with living in Sweden) components of celiac disease.
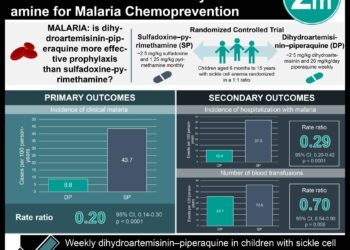
Myeloablative allogeneic hematopoietic stem cell transplantation (HSCT), curative at a rate of 95% in children with sickle cell disease, remains unsafe for adults. A small fraction of children who have undergone HSCT have unintentionally demonstrated a bone marrow mixture of both recipient and donor cells (mixed chimerism), which has proved sufficient for production of donor-type red blood cells and reversion of the sickle cell disease phenotype in the absence of graft-vs-host disease. Thus, a nonmyeloablativeregimen which intentionally creates a mixed chimerism may facilitate safer application of HSCT to adults. In this study, 30 adult patients with severe sickle cell disease underwent a nonmyeloablative bone marrow transplant. Eighty-seven percent of patients had long-term stable donor engraftment without acute or chronic graft-vs-host disease and half of engrafted patients discontinued immunosuppression medication with continued stable donor chimerism and no graft-vs-host disease. Hospitalization rates(mean) decreased from 3.23 (95% CI, 1.83-4.63) prior to HSCT to 0.63 (95% CI, 0.26-1.01) the first year after, 0.19 (95% CI, 0-0.45) the second year after, and 0.11 (95% CI, 0.04-0.19) the third year after transplant. Adverse events included pain, infections, abdominal events, and sirolimus related toxic effects. Although further long-term data are needed, this study currently shows that nonmyeloablative HSCT can successfully create a mixed-donor chimerism, allowing for complete replacement with circulating donor red blood cells.
Effect of Everolimus on Survival in Advanced Hepatocellular Carcinoma After Failure of Sorafenib
With a median overall survival of less than 1 year, patients with advanced hepatocellular carcinoma (HCC) are in need of new effective therapies. The multikinase inhibitor sorafenib is currently the only therapy shown to transiently and modestly improve overall survival in advanced HCC; however, its discontinuation rate is high secondary to adverse events. The mTOR pathway is a key regulator of cellular growth, proliferation, angiogenesis, and survival and is activated in up to 45% of HCC. Everolimus, amTOR inhibitor, prevented tumor progression and improve survival in preclinical models and was shown in early-phase clinical studies to have manageable safety and clinical activity. In this randomized, double-blind, phase 3 study, 546 adults with stage B or C HCC and Child-Pugh A liver function who failed sorafenib treatment were randomized to everolimus or placebo. At the time of analysis, 1-3 years post-treatment, 83.7% of patients in the everolimus group compared to 82.1% in the placebo group had died (hazard ratio [HR], 1.05; 95% CI, 0.86-1.27; P = .68; median overall survival, 7.6 months with everolimus, 7.3 months with placebo). The most common adverse events for everolimus vs placebo were anemia (7.8% vs 3.3%, respectively), asthenia (7.8% vs 5.5%, respectively), and decreased appetite (6.1% vs 0.5%, respectively). Given no significant difference in overall survival and slightly increased adverse events, everolimus is not recommended at this time for patients with advanced HCC.
Image: PD
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.