2 Minute Medicine Rewind May 29, 2017
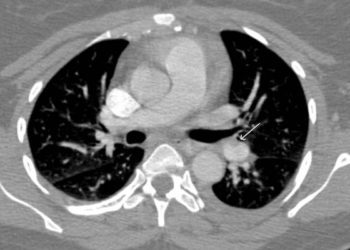
The clinical diagnosis of pulmonary embolism can be non-specific and, as such, requires objective testing. Most often, computed tomography pulmonary angiography (CTPA) is used in confirming PE. However, the incidence of PE has decreased, and with increasing accessibility to CTPA, clinicians may be overdiagnosing PE. This places patients at risk of unnecessary exposure to radiation and CT contrast. Recognizing the associated risks of CTPA, diagnostic algorithms have been developed for suspected acute PE, although adherence to these guidelines is variable in clinical practice. In this prospective cohort study, the YEARS algorithm was applied in 3465 patients with suspected acute pulmonary embolism to evaluate this novel diagnostic tool. The YEARS clinical decision rule consists of 3 items: clinical signs of deep vein thrombosis, hemoptysis and whether pulmonary embolism is the most likely diagnosis, in addition to D-dimer concentrations. In patients without YEARS items and D-dimer <1000 ng/mL, or in patients with one or more YEARS items and D-dimer <500 ng/mL, pulmonary embolism was considered excluded. All other patients underwent CTPA. Of the 3465 patients that met study inclusion criteria, PE was ruled out in 2946 patients (85%) at baseline and went untreated. Of these patients, 18 were later diagnosed with symptomatic venous thromboembolism during 3-month follow-up, with an incidence of 0.61% (95% CI 0.36 to 0.96). In this subgroup, the incidence of fatal PE was 0.20% (95% CI 0.07 to 0.44). In comparing the results of the YEARS algorithm with the widely used Wells’ rule, the authors found that CTPA was not indicated in 1651 (48%) of patients with the YEARS algorithm, compared to 1174 patients (34%) if the Wells’ rule and fixed D-dimer threshold <500ng/mL would have been applied. This study therefore shows that PE may be safely excluded using the YEARS diagnostic algorithm in patients with suspected acute PE.
Intra-arterial treatment (IAT) for acute ischemic stroke caused by proximal intracranial occlusion of the anterior circulation is both safe and effective. This is most often accomplished using a retrievable stent. However, vascular abnormalities (i.e. stenosis or occlusion) of the extracranial internal carotid artery pose a challenge during attempted IAT, as thrombi may become dislodged from an unstable plaque in a stenotic area, for example. In this subgroup analysis of a randomized controlled trial in which patients with acute ischemic stroke caused by proximal intracranial arterial occlusion were randomized to receive IAT or no IAT, 476 patients were followed up to examine whether the presence of extracranial carotid disease (ECD) modifies the effect IAT for intracranial proximal anterior circulation occlusion. The primary outcome was measured using the modified 7-point Rankin Scale (mRS) score at 90 days of follow-up, where a score of 2 or less indicates functional independence. Researchers found that a positive shift in the distribution on the mRS was observed in favour of the intervention group receiving IAT in patients with ECD (OR 3.1, 95% CI 1.7 to 5.8) and without ECD (OR 1.3, 95% CI 0.9 to 1.9). There was also no significant modification of treatment effect on the primary outcome by presence of ECD (p=0.07). This study therefore shows that IAT may be effective in patients with ECD and, as such, should be considered in complex patients with acute ischemic stroke.
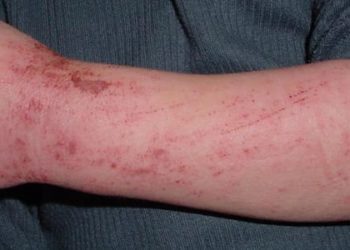
With the emergence of methicillin-resistant Staphylococcus aureus (MRSA), there has been a significant increase in the number of emergency department visits for skin infections in the United States. In diagnosing cellulitis, however, it is usually not possible to determine the exact bacterial etiology due to a lack of diagnostic specimen. Nonetheless, beta-hemolytic streptococci are presumed to be the predominant pathogens for cellulitis without purulent drainage. According to a 2006 report, MRSA was the most common cause of purulent skin infections in the US. At this time, the Infectious Diseases Society of America practice guidelines recommend that patients with cellulitis without systemic signs of infection penetrating trauma, evidence of MRSA elsewhere or injection drug use, receive an antimicrobial agent for coverage against streptococci. Despite this, however, clinicians frequently prescribe agents that include MRSA activity for cellulitis. In this randomized controlled trial, 496 patients with no wound, purulent drainage or abscess were randomized to receive cephalexin 500 mg four times daily and trimethoprim-sulfamethoxazole (TMP-SMX) 320 mg/1600 mg twice daily, or cephalexin plus placebo for 7 days in order to determine whether the addition of a MRSA-active antimicrobial yields a higher clinical cure rate of uncomplicated cellulitis than cephalexin alone. Researchers found that clinical cure rates at 14 to 21 days of follow-up were not significantly different between groups, where clinical cure occurred in 76.2% of patients in the intervention group, compared to 69.0% in the control group (difference 7.3%, 95% CI -1.0% to 15.5%, p=0.07). Between-group adverse events rates and secondary outcomes through 7 to 9 weeks did not differ significantly. This study therefore shows that among patients with uncomplicated cellulitis, the addition of TMP-SMX for coverage of MRSA may not result in higher rates of clinical resolution of cellulitis.
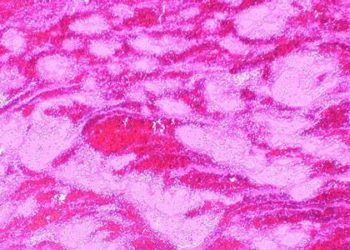
Gadolinium-based contrast agents (GBCA) provide critical clinical information not often apparent on unenhanced MRI or other imaging modalities. However, studies have demonstrated the unexpected accumulation of gadolinium within the neural tissues of adults following intravenous GBCA exposure, prompting further research into the safety and toxicity of these agents. The extent of deposition in children exposed to GBCA and whether there is a component of age-dependent breakdown of the blood-brain barrier is unclear. In this retrospective case-control study, post-mortem brain tissues of 3 pediatric patients that received at least 4 gadodiamide-enhanced MRI examinations were compared with 3 pediatric patients who did not receive any gadolinium-enhanced MRI examinations during their lifetime to address this knowledge gap. Researchers found that elemental gadolinium could be detected in all neuroanatomic regions among gadodiamide-exposed patients, with the highest concentrations detected in the dentate nucleus or pons. No gadolinium was detected in control patients. In the patients that were exposed, deposition in the dentate tissues was not associated with any pathologic changes. However, dentate tissue from the 2 patients that received the highest cumulative dose of gadodiamide had mild to severely gliotic regions with prominent axonal spheroids, which the authors indicate may be due to the primary exposure or prior external beam radiation. This research therefore confirms the presence of intracranial gadolinium deposits in pediatric patients following exposure to intravenous GBCA for MRI. This has important implications for development, as pediatric brains are more susceptible to the neurotoxic effects of heavy metal exposure.
Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome
Dravet syndrome is a complex childhood epilepsy disorder associated with drug-resistant seizures, and therefore, a high mortality rate. This rare genetic form of epileptic encephalopathy is primarily due to a loss of function mutation in the SCN1A gene. Recent media reports have generated interest in the use of cannabidiol for the treatment of epilepsy in these patients. However, research performed in this area thus far has demonstrated mixed results. In this randomized controlled trial, 120 children and young adults with Dravet syndrome and drug-resistant seizures were randomized to receive either a cannabidiol oral solution (20 mg/kg/day) or placebo, in addition to standard antiepileptic treatment in order to study the effectiveness of adjunctive cannabidiol in the treatment of drug-resistant seizures in Dravet syndrome.
GW Pharmaceuticals, who was responsible for trial design with input from investigators and other experts, trial management, site monitoring, trial pharmacovigilance, data analysis, and statistical analysis, funded this study. Researchers found that the median frequency of convulsive seizures per month decreased from 12.4 to 5.9 with the use of cannabidiol compared to a decrease from 14.9 to 14.1 in the placebo group (median difference -22.8%, 95% CI -41.4 to -5.4, p=0.01). The frequency of total seizures was also significantly reduced with cannabidiol p=0.03), however there was no significant reduction in non-convulsive seizures, specifically. In addition, there was no significant difference with respect to the percentage of patients who achieved least a 50% reduction in convulsive seizure frequency (OR 2.00, 95% CI 0.93 to 4.30, p=0.08). Adverse events and withdrawals from the study occurred more frequently in the intervention group. This study therefore indicates that the use of cannabidiol may result in a greater reduction in the frequency of convulsive seizures in patients Dravet syndrome compared to placebo.
Image: PD
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.