2 Minute Medicine Rewind October 28 – November 3, 2013
Image: PD
In this section, we will highlight the key high-impact studies, updates, and analyses published in medicine during the past week.
Non-publication of large randomized clinical trials: cross sectional analysis
Efforts, including the listing of industry affiliations and the listing of trials on clinicaltrials.gov, have been made to improve the transparency and accountability of medical research. In this retrospective analysis of trials listed on clinicaltrials.gov, investigators analyzed clinical trials with greater than 500 participants between November 2009 and November 2012. 585 clinical trials were identified, of which 171 (29%) trials with 299,763 participants remain unpublished. Trials sponsored by industry were more likely to go unpublished (150 (38%) vs. 21 (18%), p = 0.003). The majority (78%) of registered trials with unpublished results also did not have results available on clinicaltrials.gov. The authors concluded that despite exposing many participants to potential hazards, non-publication of results was common and online databases had limited data from unpublished trials.
Bivalirudin Started during Emergency Transport for Primary PCI
Bivalirudin has been previously studied in the open-label Horizons-AMI trial and linked with decreased 3 year rates of mortality, cardiac mortality, and major bleeding compared to heparin and IIb/IIIa inhibitor in patients with ST elevation myocardial infarctions (STEMIs). In this randomized, open-label, controlled trial, 2218 patients from nine European countries with STEMIs were randomized to bivalirudin or heparin and IIb/IIIa inhibitors in the ambulance or prior to transfer to PCI performing hospital. At thirty days, there was no difference in the rates of overall death or cardiac death. The bivalirudin arm had a decreased rate of non-CABG major bleeding (2.6% vs. 6.0%, p < 0.001), however it also had more frequent stent thrombosis (1.6% vs. 0.5%, p = 0.02). These results are consistent with prior literature, however this study of pre-hospital bivalirudin did not have the difference in 30 day mortality seen in the Horizons-AMI trial.
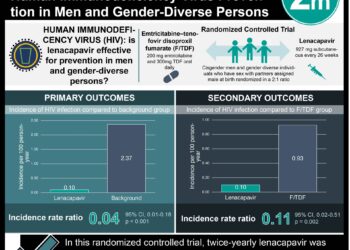
While efforts have been made to increase the ease and convenience of rapid HIV testing, the efficacy of HIV risk-reduction counseling remains unclear. In this randomized trial of 5012 patients from nine sexually transmitted disease (STD) clinics, participants were randomized to rapid HIV test with risk reduction counseling or rapid HIV test with information alone and assessed for sexually transmitted infections (STI) at 6 months. There was so significant difference in 6-month composite STI incidence at 6 months (12.3% vs. 11.1%, 95% CI 0.94-1.33). Risk reduction counseling in conjunction with rapid HIV test did not significantly affect STI incidence among STD clinic patients.
Autologous Transplantation as Consolidation for Aggressive Non-Hodgkin’s Lymphoma
Autologous stem cell transplant is not considered a common therapy for initial remission for patients with aggressive non-Hodgkin’s lymphoma. In this multicenter, randomized, controlled trial, patients with high or high-intermediate risk non-Hodgkin’s lymphoma were treated with five cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP plus rituximab. 253 patients with a response were further randomized to either 3 more cycles of induction chemotherapy or one cycle of induction chemotherapy and autologous stem cell transplant. Patients in the transplantation arm had a higher rate of 2-year progression free survival (69% vs. 55%, p = 0.005), but similar rates of 2-year overall survival (74% vs. 71%, p = 0.30). Subset analysis of just high risk patients suggested an improvement of 2-year overall survival (82% vs. 64%). Early transplant could be a potential therapy for non-Hodgkin’s lymphoma.
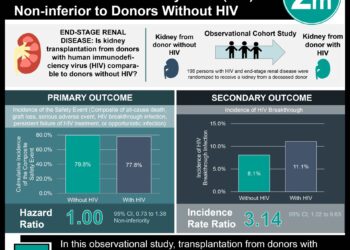
Autosomal dominant polycystic kidney disease is a chronic progressive disease that results in gradual enlargement of kidneys, formation of cysts, and ultimately progresses to end stage renal disease with no known therapy for improvement. In this randomized, multicenter, single blinded, controlled trial, 75 patients were randomized to long acting release (LAR) octreotide or placebo. At one year, there were less total kidney volume increase in the octreotide arm compared to the placebo arm (46.2mL vs. 143.7mL, p = 0.032). At three years, there was a non-statistically significant difference in kidney volume increase (220.1mL vs. 454.3mL, p = 0.25). Further studies should be performed to assess the utility of octreotide in managing autosomal dominant polycystic kidney disease.
By David Ouyang
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT. Content is produced in accordance with fair use copyrights solely and strictly for the purpose of teaching, news and criticism. No benefit, monetary or otherwise, is realized by any participants or the owner of this domain.