In-utero exposure to tenofovir-emtricitabine not associated with higher risk of adverse birth outcomes
1. Pairwise comparisons of tenofovir-emtricitabine and ritonavir-boosted lopinavir (TDF-FTC-LPV/r), zidovudine-lamivudine and ritonavir-boosted lopinavir (ZDV-3TC-LPV/r), and tenofovir-emtricitabine and ritonavir-boosted atazanavir (TDF-FTC-ATV/r) HIV regimens among pregnant women with HIV showed all three regimens had comparable risks of preterm birth, low birth weight, and neonatal death.
2. There was a higher risk of preterm birth, low birth weight, and adverse birth events noted among women who initiated any of the regimens prior to conception.
Evidence Rating Level: 2 (Good)
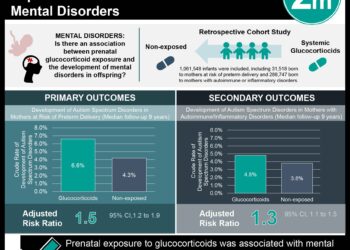
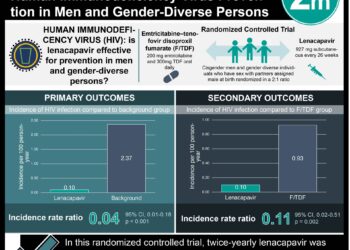
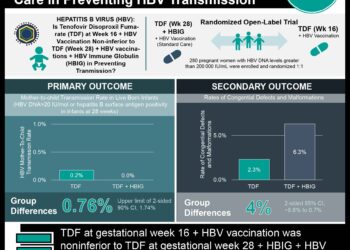
Study Rundown: Perinatal transmission of human immunodeficiency virus (HIV) has been greatly reduced by the introduction of antiretroviral (ART) therapy. There are several variations in ART therapy, and studies continue to explore which regimen options work best for specific subpopulations of HIV-infected mothers. A notable previous study found a greater risk of for premature infant birth and death in pregnant HIV patients taking ZDZ-3TC-LPV/r. The current analysis used data from two large United States based perinatal cohorts to study the comparative outcomes of tenofovir-emtricitabine and ritonavir-boosted lopinavir (TDF-FTC-LPV/r) versus zidovudine-lamivudine and ritonavir-boosted lopinavir (ZDV-3TC-LPV/r) versus tenofovir-emtricitabine and ritonavir-boosted atazanavir (TDF-FTC-ATV/r) on incidences of premature birth, low birth weight, or neonatal death. No substantial differences in premature birth, low birthweight, or neonatal death were found when comparing the three regimens, TDF-FTC-LPV/r to ZDV-STC-LPV/r to TDF-FTC-ATV/r. There was however, a higher risk of preterm birth, low birth weight, and adverse outcomes noted among women who initiated TDF-FTC-LPV/r before conception compared to women that initiated the other drug regimens before conception.
Strengths of this analysis include its use of two broad comprehensive databases, however limitations included small cohort numbers for certain study parameters, which limited the study’s power and its ability to calculate statistically significant differences.
Click to read the study, published in NEJM
Relevant Reading: Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis
In-Depth [retrospective cohort]: This retrospective cohort study compared data from the Surveillance Monitoring for ART Toxicities (SMARTT) study of the Pediatric HIV/AIDS Cohort Study (PHACS) and the P1025 study of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network. A total of 4646 mother-infant birth outcomes were included across both cohorts, corresponding to 3847 unique women. There were 128 mother-infant pairs (2.8%) that were exposed to TDF-FTC-LPV/r, 539 (11.6%) pairs exposed to TDF-FTC-ATV/r, and 954 (20.5%) pairs exposed to ZDV-3TC-LPV/r. Comparative analyses of TDF-FTC-LPV/r to ZDV-3TC-LPV/r showed that there was no increased risk of preterm birth (relative risk [RR], 0.90; 95% confidence interval [CI], 0.60 to 1.33), low birth weight (RR, 1.13; 95% CI, 0.78 to 1.64) or overall adverse events (RR, 0.92; 95% CI, 0.67 to 1.28) between groups. Similar findings showing no difference in any of the adverse outcomes were noted when TDF-FTC-LPV/r was compared to TDF-FTC-ATV/r. Subgroup analyses looking at women with their first singleton pregnancy or women who did not change ART regimens during therapy showed no significant change in RR when comparing ART regimens. Women who initiated any of the regimens prior to pregnancy had greater risks of preterm birth, very low birth weight, and severe adverse outcomes compared to women initiating therapy in their second or third trimester.
Image: PD
©2018 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.