COVID-19 mRNA-1273 vaccine safe and immunogenic in phase 1 trial
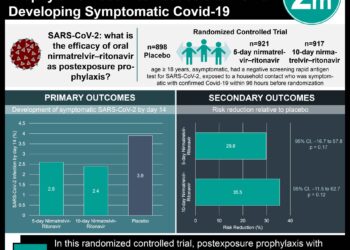
1. The mRNA-1273 SARS-CoV-2 (COVID-19) vaccine was shown to be safe and immunogenic amongst the 45 COVID-19 negative participants in this phase 1 clinical trial, when compared to serum convalescent plasma
2. Only patients in the highest 2 dosage groups experienced a severe febrile episode after the 2nd dose was administered.
Evidence Rating Level: 2 (Good)
Study Rundown: The mRNA-1273 SARS-CoV-2 (COVID-19) vaccine, which encodes a stabilized perfusion spike trimer (S-2P), was designed and tested in a phase 1 clinical trial 66 days after the release of the COVID-19 genomic sequence. It was designed and manufactured by Moderna and was administered as a 0.5ml injection into the deltoid muscle across two administrations (day 1 and 29). A total of 45 participants received at least one of the two injections with all participants able to attend scheduled clinical follow up. Amongst phase 1 participants, the 2-dose administered vaccine was completed without serious toxicity. A majority of the known reactogenicity occurred after the second dose was administered in 250-microgram group (with most reporting a post injection fever). The mRNA-1273 vaccine was immunogenic and caused binding antibody IgG to rapidly increase such that all were seroconverted by day 15. All participants developed a binding antibody response to both full-length S-2P and receptor-binding domains. This response was similar to the effects seen in convalescent serum gathered from patient’s previously infected with COVID-19.
Click to read the study in NEJM
Relevant Reading: Draft landscape of COVID-19 candidate vaccines—World Health Organization
In-Depth [prospective cohort]: This phase 1, dose-escalation, open-label clinical trial research study aimed to assess the safety, reactogenicity and immunogenicity of the mRNA-1273 COVID-19 vaccine. Patients were between 18 and 55 and received 2 injections 28 days apart at 25 micrograms, 100 micrograms or 250 micrograms dosage. The candidate vaccine encoded the S-2P antigen which consist of the glycoprotein of the COVID-19 with a transmembrane anchor and intact cleavage sites at S1-S2. Dosage days were day 1 and 28 with follow up occurring 7- and 14-days post injection and days 57, 119, 209, and 394. Binding antibody responses were assessed via ELISA, PsVNA, and PRNT assays on specimens collected from all participants on days 1, 15, 29, 36, 43, 57. Reactogenicity was assessed by exploring via an intracellular cytokine-staining assay. Study findings noted that 5 members of the 25 microgram group, 10 in the 100 microgram group and 8 in the 250 microgram group had systemic adverse effects that were mild or moderate after the first administration and after the 2nd administration, 7/14 for the 25 microgram group and all of the 100 microgram and 250 microgram group reports systemic adverse effects. Binding antibodies increased rapidly after the first administration of each vaccine and after the 2nd administration all participants had detected PsVNA response in serum samples. The 25 microgram and 100 microgram dose group elicited CD4 T-cell response that led towards expression of Th1 cytokines.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.