#VisualAbstract: Dolutegravir noninferior to a ritonavir-boosted protease inhibitor regimen for treating HIV
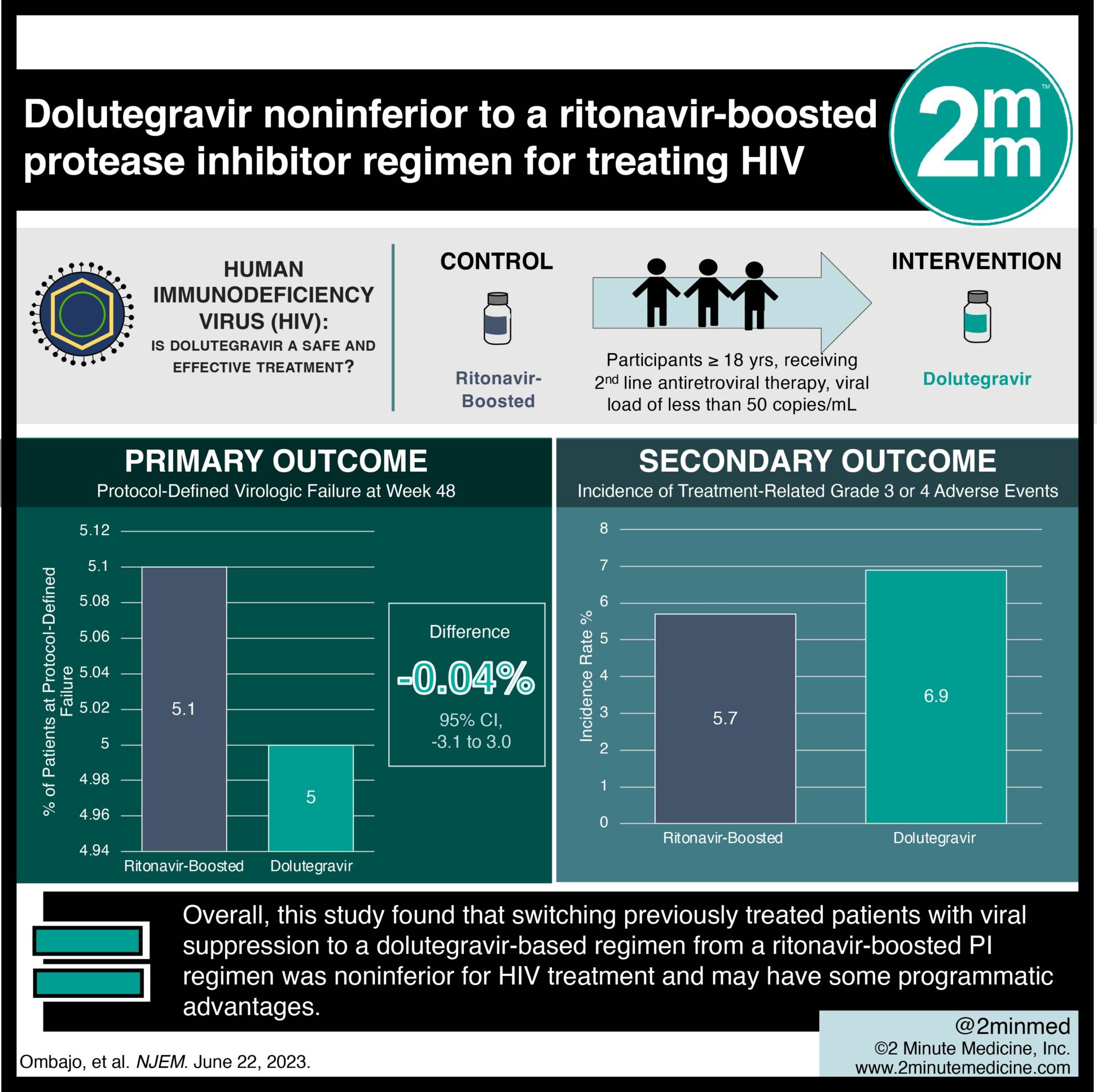
1. In the randomized controlled trial, dolutegravir treatment was noninferior to a regimen containing a ritonavir-boosted protease inhibitor (PI) for treating human immunodeficiency virus (HIV).
2. Dolutegravir treatment for HIV was not associated with an increased risk of adverse events as compared to ritonavir-boosted treatment.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Currently, the World Health Organization recommends that people living with HIV who have treatment failure with two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) be switched to a regimen containing alternative NRTIs and a ritonavir-boosted PI. This recommendation was changed to favor the initiation of a second-line regimen containing dolutegravir and two NRTIs. However, there is a gap in knowledge as to understanding the efficacy of switching previously treated adults with viral suppression who had not received an integrase inhibitor previously, who were receiving a ritonavir-boosted PI plus NRTIs, and for whom no genotype information on drug-resistance mutations was available to receive dolutegravir plus NRTIs. Overall, this study found that switching previously treated patients with viral suppression to a dolutegravir-based regimen from a ritonavir-boosted PI regimen was noninferior for HIV treatment and may have some programmatic advantages. This study was limited by the trial being open-label by design and children/adolescents being excluded from the trial. Nevertheless, these study’s findings are significant, as they demonstrate that a dolutegravir-based regimen is noninferior to a ritonavir-boosted PI regimen for the treatment of HIV.
Click to read the study in NEJM
Relevant Reading: Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children
In-Depth [randomized controlled trial]: This prospective, multicenter, open-label trial was conducted at four sites in Kenya over 48 weeks. Patients who were at least 18 years of age, had been receiving second-line antiretroviral therapy containing two NRTIs and a ritonavir-boosted PI for at least 24 weeks, and had had a viral load of less than 50 copies per milliliter for at least 12 weeks before enrollment and at the time of enrollment were eligible for the study. Patients who had previous exposure to an integrase strand-transfer inhibitor, pregnancy or breastfeeding, and baseline conditions that would result in a regimen change, such as advanced kidney or liver disease and grade three or four lipid abnormalities were excluded from the study. The primary outcome measured was protocol-defined virologic failure (defined as two consecutive measurements of an HIV-1 RNA viral load of ≥50 copies per milliliter) at week 48, as assessed by means of the Food and Drug Administration snapshot analysis. Outcomes in the primary analysis were assessed via the intention to treat protocol with 95% confidence intervals to assess noninferiority. Based on the primary analysis, at week 48, 20 participants (5.0%) in the dolutegravir group and 20 patients (5.1%) in the ritonavir-boosted PI group met the primary endpoint (difference, −0.04%; 95% confidence interval, −3.1 to 3.0), a result that met the criterion for noninferiority. No mutations conferring resistance to dolutegravir or the ritonavir-boosted PI were detected at the time of treatment failure. The incidence of treatment-related grade three or four adverse events was similar in the dolutegravir group and the ritonavir-boosted PI group. In summary, this study demonstrates that dolutegravir treatment was noninferior to a regimen containing ritonavir in previously treated HIV patients with viral suppression.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.