Tiragolumab With Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage SCLC
1. Median PFS and OS between the tiragolumab and control arms were similar
2. Treatment-related AEs that were grade 3/4 occurred in 52.7% in the tiragolumab arm and 55.7% in the control arm.
Evidence Rating Level: 1 (Excellent)
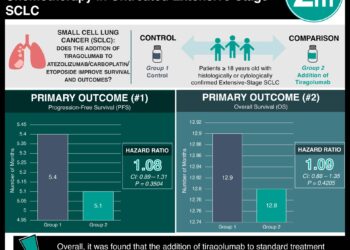
Study Rundown: Extensive-stage small-cell lung cancer (ES-SCLC) is currently treated with atezolizumab/carboplatin/etoposide or durvalumab/chemotherapy, however, most patients have poor median survival. Tiragolumab, an antibody targeting T-cell immunoreceptor with Ig and ITIM domains (TIGIT), has shown antitumor activity. This study evaluated the addition of tiragolumab to atezolizumab/carboplatin/etoposide in untreated ES-SCLC patients. The primary endpoints were progression-free survival (PFS) and overall survival (OS), and secondary endpoints included objective response rate (ORR), duration of response (DoR), and safety. Median PFS was 5.1 months in the tiragolumab group vs 5.4 months in the control group, with HR 1.08, with similar results in a sub-group analysis in patients without brain metastases. PFS at 6 and 12 months were 31.3% and 12.3% with tiragolumab and 38.0% and 14.1% with control. Median OS was 12.8 months in the tiragolumab group vs 12.9 months in the control group, with HR 1.09 (p=.4205), with similar results in a sub-group analysis in patients without brain metastases, or with patients with positive PD-L1. OS at 24 months was 21% vs 26%, respectively. ORR was 70.8% and 65.6% with tiragolumab and control, respectively, and the median DoR was 4.2 and 5.1 months. With regards to safety, treatment-related AEs (TRAEs) that were grade 3/4 occurred in 52.7% in the tiragolumab arm and 55.7% in the control arm and were most commonly anemia and neutropenia. The strengths of this study included its methodology and the inclusion of patients with brain metastasis, and the limitations included a small sample size. Overall, it was found that the addition of tiragolumab to standard treatment (atezolizumab/carboplatin/etoposide) did not improve outcomes in patients with extensive-stage small-cell lung cancer.
Click to read the study in JCO
In-Depth [randomized controlled trial]: This international phase III trial recruited adults with treatment-naïve confirmed ES-SCLC and randomized (1:1) them to tiragolumab (243 patients) vs placebo (247 patients), in addition to atezolizumab/carboplatin/etoposide. Patients with brain metastases were permitted. Median PFS was 5.1 months in the tiragolumab group vs 5.4 months in the control group, with HR 1.08 (95%CI, 0.89-1.31) with similar results in a sub-group analysis in patients without brain metastases. PFS at 6 and 12 months were 31.3% and 12.3% with tiragolumab and 38.0% and 14.1% with control. Median OS was 12.8 months in the tiragolumab group vs 12.9 months in the control group, with HR 1.09 (95%CI, 0.88-1.35, p=.4205), with similar results in a sub-group analysis in patients without brain metastases, or with patients with positive PD-L1. OS at 24 months was 21% vs 26%, respectively. ORR was 70.8% and 65.6% with tiragolumab and control, respectively, and the median DoR was 4.2 and 5.1 months. With regards to safety, TRAEs that were grade 3/4 occurred in 52.7% in the tiragolumab arm and 55.7% in the control arm and were most commonly anemia and neutropenia. Overall, it was found that the addition of tiragolumab to standard treatment (atezolizumab/carboplatin/etoposide) did not improve outcomes in patients with extensive-stage small-cell lung cancer.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.