#VisualAbstract Amivantumab Plus Lazertinib Improves Survival in EGFR-mutated Advanced NSCLC
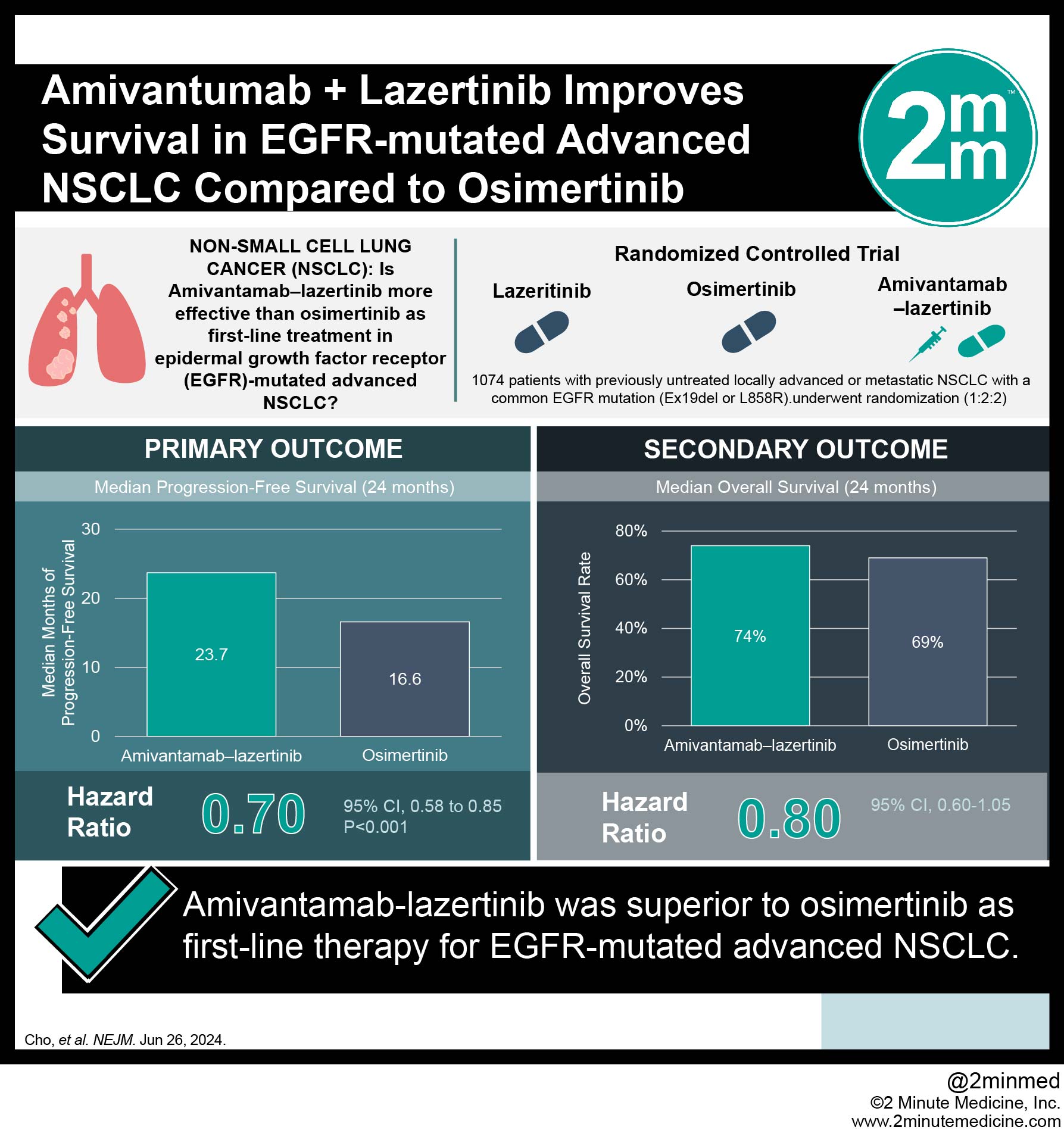
1. In this randomized trial, amivantamab-lazertinib led to significantly longer progression-free survival than osimertinib therapy for mutated advanced non-small-cell lung cancer (NSCLC).
2. Amivantamab-lazertinib showed superior efficacy to osimertinib as first-line therapy for epidermal growth factor receptor (EGFR) mutated advanced NSCLC.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Approximately 15 to 50% of non-squamous advanced NSCLC have an activating mutation in the EGFR gene. Of these EGFR mutations, approximately 90% of them are exon 19 deletions (Ex19del) or exon 21 codon p.Leu858Arg (L858R) substitutions. Currently, the first-line therapy for Ex19del and L858R advanced NSCLC is osimertinib, which is a third-generation EGFR tyrosine kinase inhibitor (TKI). However, resistance to third-generation EGFR TKI develops in nearly all patients, mostly through secondary EGFR pathway alteration and MET pathway activations. Amivantamab is an EGFR MET bispecific antibody with an immune cell-directing activity that has shown progression-free survival when used with chemotherapy for NSCLC. Lazertinib, a highly selective central nervous system penetrant third-generation EGFR TKI, has also shown efficacy in improving progression-free survival in EGFR-mutated advanced NSCLC. This phase three clinical trial assessed the efficacy and safety of amivantamab-lazertinib compared to osimertinib as a first-line treatment in patients with EGFR-mutated advanced NSCLC. Results from this study found that the amivantamab-lazertinib group had significantly longer progression-free survival than the osimertinib group. Overall, amivantamab-lazertinib showed superior efficacy to osimertinib as a first-line therapy for advanced EGFR-mutated NSCLC.
Click here to read the study in NEJM
In-Depth [randomized controlled trial]: This phase three clinical trial assessed and compared the safety and efficacy of amivantamab-lazertinib to osimertinib as a first-line therapy for advanced EGFR mutated NSCLC. Adult patients with previously untreated locally advanced or metastatic EGFR mutated (Ex19del or L858R) NSCLC were eligible to participate in this trial. A total of 1,074 patients were randomly assigned in a 2:2:1 ratio to either the amivantamab-lazertinib group (n=429), the osimertinib group (n=429), or the lazertinib group (n=216). Amivantamab was administered intravenously weekly at a dose of 1050mg for the first four weeks. For the second cycle, the same dose was administered every two weeks. Oral Osimertinib (80mg) and lazertinib (240mg) were taken daily. The primary endpoint was progression-free survival in the amivantamab-lazertinib compared to the osimertinib group. Results from this study found that the median progression-free survival was significantly longer in the amivantamab-lazertinib group than the osimertinib group (23.7 vs. 16.6 months; hazard ratio for disease progression or death, 0.70; 95% Confidence Interval [CI], 0.58 t 0.85; p<0.001). An objective response was seen in 86% of the patients in the amivantamab-lazertinib group (95% CI, 83 to 89) and 85% of those in the osimertinib group (95% CI, 81 to 88). The main adverse effects were EGFR-related toxic effects. Discontinuation of the treatment agents due to treatment-related adverse effects was 10% in the amivantamab-lazertinib group and 3% in the osimertinib group. In summary, results from this study found that amivantamab-lazertinib had superior efficacy to osimertinib as a first-line treatment for EGFR-mutated advanced NSCLC.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.