The TRINOVA-1 trial: trebananib decreases progression of recurrent ovarian cancer
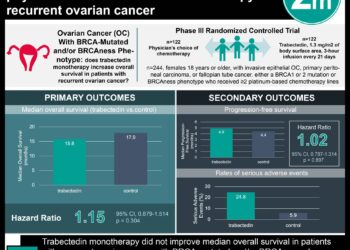
1. Ovarian cancer patients randomized to receive paclitaxel + trebananib had improved progression-free survival versus paclitaxel + placebo.
2. Patients randomized to receive paclitaxel + trebananib demonstrated improved RECIST 1.1 and CA-125 scores.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Epithelial ovarian cancer, which includes ovarian, fallopian tube, and primary peritoneal cancer, is one of the most lethal gynecological malignancies. While response to first-line platinum- and taxane-based chemotherapeutic agents is good, many women experience lethal recurrence. For platinum-resistant cancers, current guidelines recommend sequential single agent therapy with paclitaxel and pegylated liposomal doxorubicin. Combination regimens that incorporate anti-angiogenic agents, such as bevacizumab, have also demonstrated benefit but anti-VEGF agents have been associated with significant adverse side effects such as hypertension, thrombosis, bleeding and bowel perforations. This study is the first to evaluate the effectiveness of an anti-angiogenic agent (Trebananib) that works outside the VEGF pathway, binding Angiopoietin-1 and -2 and blocking binding to the Tie2 receptor. Because it does not alter the VEGF pathway, trebananib is thought to have fewer anti-VEGF-associated adverse effects. In the present work, authors found that paclitaxel + trebananib was superior to paclitaxel + placebo in increasing progression-free survival and yielded comparable results in treating platinum-resistant ovarian cancer compared to previously published effects of single agent + bevacizumab.
Limitations include loss of patients to discontinuation and potential bias from involvement of the study funder in creation of the trial design, data collection and analysis. Future randomized controlled trials comparing the efficacy of a single agent + trebananib as compared to single agent + bevacizumab are needed to determine its superiority over current combination treatment options.
Click to read the study in The Lancet Oncology
Click to read an accompanying editorial in The Lancet Oncology
Relevant Reading: Latest research and treatment of advanced-stage epithelial ovarian cancer
In-Depth [randomized controlled trial]: This study evaluated the effectiveness of trebananib, an anti-angiogenic agent, as an adjunct to paclitaxel for treatment of platinum-resistant, recurrent epithelial ovarian, fallopian tube and peritoneal cancer. Outcomes evaluated included progression-free survival, overall survival, objective response as measured by CA-125, patient-reported outcomes and adverse events.
Women randomized to receive trebananib had longer progression-free survival (7.2 months vs. 5.4 months, p<0.0001) but did not demonstrate improved overall mortality compared to women randomized to receive placebo. The trebananib group also demonstrated superior objective response to treatment (p<0.0071). Patients in the trebananib group were more likely to discontinue treatment due to adverse events (edema was the principle adverse side effect).
More from this author: Contraceptive use before first intercourse linked with improved reproductive outcomes, HPV catch-up vaccine completion low among pregnant and black women, Low screening rates for smokeless tobacco use in pregnant women, Expectant management may be appropriate in ectopic pregnancy, Decreased HIV co-receptor CCR5 expression after IUD insertion
Image: PD
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.



![Childhood ADHD associated with increased risk of suicide [Physician Comment]](https://www.2minutemedicine.com/wp-content/uploads/2013/03/PET-image1-e1377449984183-75x75.jpg)