Abemaciclib linked to disease control in patients with p16ink4A-negative mesothelioma
1. Disease control was reported in over half of the patients treated with abemaciclib
2. Most common adverse events included fatigue, diarrhea, nausea, lower appetite, and anemia
Evidence Rating Level: 2 (Good)
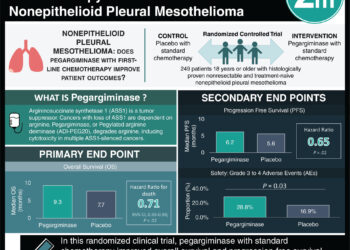
Study Rundown: Deletions in the CDKN2A tumour suppressor gene are often found in patients with mesothelioma. Restoration of p16ink4A (a cyclin-dependent kinase (CDK) 4 and 6 inhibitor that is encoded by CDKN2A) in such patients has previously led to suppression of mesothelioma in preclinical models. This study explored the use of abemaciclib, a CDK4 and CDK6 inhibitor, in patients with p16ink4A-deficient mesothelioma who had progressed on a platinum-based chemotherapy regimen. Patients were only assigned to a treatment group. The primary endpoint was disease control rate at 12 weeks, which was defined as patients having stable disease or partial response to treatment. Disease control was observed in over half of the patients. Median progression-free and overall survival were calculated through post-hoc exploratory analysis. Patients were also stratified based on the presence of MTAP deletion (a gene frequently co-deleted with CDKN2A in mesothelioma) among certain patients. MTAP-negative patients with mesothelioma showed a greater tumour volume reduction with abemaciclib than MTAP-positive patients. Adverse events of all grades were observed. Some of the most common adverse events were diarrhea, nausea, lower appetite, fatigue, and anemia. Limitations to this study include its low generalizability due to its small sample size and predominantly male patients, as well as the absence of a comparison group. The strength of this study is that it suggested abemaciclib had a clinical benefit to p16ink4A-deficient mesothelioma patients which remains an area of unmet need. Overall, this study showed promise for abemaciclib as a treatment option in p16ink4A-negative patients with relapsed mesothelioma.
Click to read the study in The Lancet Oncology
Relevant Reading: Systemic treatments for mesothelioma: standard and novel
In-Depth [prospective cohort]: This phase II, single-arm, open-label clinical trial in the United Kingdom included 26 patients with p16ink4A-negative mesothelioma who had progressed on a platinum-based chemotherapy regimen. Disease control at 12 weeks was achieved in 54% of patients (95% confidence interval [CI], 36 to 71). 42% of patients had stable disease (95% CI, 26 to 60) and 12% had an observed partial response (95% CI, 3 to 27). Through post-hoc exploratory analysis, median progression-free survival was 128 days (95% CI, 105 to 252) and overall survival was 217 days (95% CI, 111 to 387). A total of 11 patients had an MTAP gene deletion and post-hoc exploratory analysis of these patients showed a median change in tumour volume of -18% when compared to MTAP-positive patients (IQR -17 to -26). Adverse events of each grade were observed. The most common adverse events were fatigue (62%), nausea (35%), lower appetite, (31%), diarrhea (50%) and anemia (31%). Overall, abemaciclib led to disease control in over 50% of p16ink4A-negative patients with relapsed mesothelioma and may be a promising treatment option.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.