Adjuvant everolimus does not improve survival for patients with resected renal cell carcinoma
1. Recurrence-free survival was significantly different between the adjuvant everolimus and placebo groups.
2. Grade 3 or higher adverse events were more frequent in the everolimus group with oral mucositis being the most reported within this treatment arm.
Evidence Rating Level: 1 (Excellent)
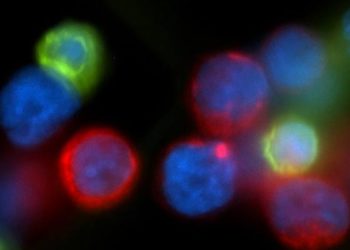
Study Rundown: Patients with renal cell carcinoma face a high risk of disease relapse after surgery. Until now, tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors have been used as systemic therapy following surgery of other solid tumours. However, there remains limited evidence surrounding their efficacy in patients with renal cell carcinoma undergoing complete resection. This randomized controlled trial aimed to evaluate the safety and efficacy of everolimus, a mammalian target of rapamycin inhibitor, in patients with surgically resected renal cell carcinoma at intermediate-high or very high risk of recurrence. The primary outcome was recurrence-free survival up to 54 weeks, while the key secondary outcome was the likelihood of grade 3 or higher adverse events. According to study results, everolimus did not significantly improve recurrence-free survival compared to placebo and was associated with an increased occurrence of serious adverse events. Although this study was well done, it was limited by a lack of statistical significance, suggesting caution in interpreting the results.
Click to read the study in The Lancet
Relevant Reading: Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma
In-depth [randomized controlled trial]: Between Apr 1, 2011, and Sept 15, 2016, 1545 patients were screened for eligibility at 398 academic and community centers in the USA. Included were patients with histologically confirmed renal cell carcinoma at intermediate-high or very high risk of recurrence post-surgical resection. Altogether, 1499 patients (755 in everolimus and 744 in placebo) were included in the final analysis. The primary outcome of recurrence-free survival demonstrated a longer but statistically nonsignificant improvement with everolimus (5-year survival 67%, 95% confidence interval [CI] 63-70%) compared to placebo (5-year survival 63%, 95% CI 60-67; stratified hazard ratio [HR] 0.85, p=0.051). A significant improvement in recurrence-free survival with everolimus was noted in the very high-risk group (HR 0.79, 95% CI 0.65-0.97, p=0.022) but not in the intermediate-high risk group (HR 0.99, 95% CI 0.73-1.35, p=0.96). Regarding safety outcomes, everolimus was associated with an increased incidence of grade 3 or worse adverse events with oral mucositis (14% for everolimus versus <1% for placebo) being the most common. Overall, findings from this study suggest that postoperative everolimus does not significantly enhance recurrence-free survival in renal cell carcinoma patients at high risk of recurrence.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![2 Minute Medicine: Pharma Roundup: Price Hikes, Breakthrough Approvals, Legal Showdowns, Biotech Expansion, and Europe’s Pricing Debate [May 12nd, 2025]](https://www.2minutemedicine.com/wp-content/uploads/2025/05/ChatGPT-Image-May-12-2025-at-10_22_23-AM-350x250.png)