Aficamten improves oxygen uptake in patients with obstructive hypertrophic cardiomyopathy
1. In this randomized controlled trial, aficamten significantly improved peak oxygen uptake in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM) compared with placebo.
2. The results for all 10 secondary endpoints were significantly improved with aficamten.
Evidence Rating Level: 1 (Excellent)
Study Rundown: HCM is one of the most common genetic heart diseases and is characterized by left ventricular hypertrophy without other obvious causes. HCM is one of the leading causes of sudden cardiac death in athletes and young, healthy individuals, and symptoms include exertional dyspnea and reduced exercise capacity. Left ventricular outflow tract (LVOT) obstruction is a strong determinant of the progression of HCM. It is induced by several mechanisms, including cardiac hypercontractility and the mitral valve’s systolic anterior motion (SAM). Established pharmacologic therapies have exhibited limited efficacy, including suboptimal reduction of symptoms and the LVOT pressure gradient. Mavacamten, a recently approved cardiac myosin inhibitor, has shown promise in improving symptoms and exercise capacity in patients with obstructive HCM. Aficamten, a reversible cardiac myosin inhibitor with a wider therapeutic window than mavacamten, recently significantly reduced LVOT gradients in patients with obstructive HCM in a phase two trial. This phase three trial evaluated the efficacy and safety of aficamten in patients with symptomatic obstructive HCM. Overall, it showed that aficamten significantly improved peak oxygen uptake in patients with symptomatic obstructive HCM compared to placebo. Moreover, the results for all 10 prespecified secondary trial endpoints were significantly improved with aficamten. The generalizability of the study findings is limited by the decreased ethnic diversity of the patient population and the relatively short treatment period explored by the trial.
Click to read the study in NEJM
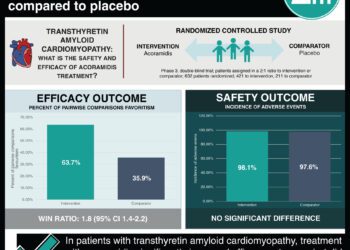
In-Depth [randomized controlled trial]: SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM) is a phase 3, double-blind, randomized, placebo-controlled trial that investigated the efficacy and safety of aficamten in patients with obstructive symptomatic HCM. Eligible patients were between 18 and 85 years of age. They had a left ventricular thickness of at least 15 mm without other discernible causes, an ejection fraction of at least 60%, and LVOT gradients of at least 30 mmHg at rest and 50 mmHg following the Valsalva maneuver. Enrolled participants were randomly assigned in a 1:1 ratio to receive aficamten or placebo once daily for 24 weeks. The primary endpoint was the change in peak oxygen uptake from baseline to week 24. The 10 secondary endpoints were defined hierarchically and included the change from baseline to week 24 in the Kansas City Cardiomyopathy Questionnaire clinical summary score, the improvement of at least one New York Heart Association (NYHA) functional class at week 24, and the change from baseline to week 24 in the LVOT gradient after the Valsalva maneuver. Overall, 282 patients underwent randomization; 142 were assigned to receive aficamten, and 140 were assigned to receive a placebo. The mean age of the trial participants was 59.1 years, the mean baseline resting LVOT gradient was 55.1 mmHg, and the mean left ventricular ejection fraction was 74.8%. The mean change in the peak oxygen uptake was 1.8 ml per kilogram per minute (95% confidence interval [CI], 1.2 to 2.3) in the aficamten group and 0.0 ml per kilogram per minute (95% CI, -0.5 to 0.5) in the placebo group (least-squares mean between-group difference, 1.7 ml per kilogram per minute; 95% CI, 1.0 to 2.4; p<0.001). The results of the 10 secondary endpoints were all significantly improved with aficamten compared to placebo. In summary, aficamten significantly improved peak oxygen uptake versus placebo in patients with symptomatic obstructive HCM.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.