Berzosertib Plus Topotecan vs Topotecan Alone in Relapsed Small Cell Lung Cancer
1 .The progression-free survival in berzosertib with topotecan vs topotecan alone was not statistically significant, but the overall survival was significant.
2. Grade ≥3 treatment-related adverse events were similar between both groups.
Evidence Rating Level: 1 (Excellent)
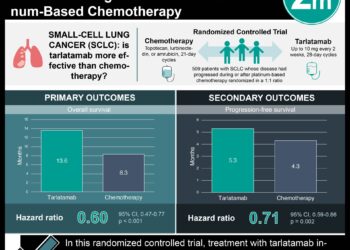
Study Rundown: Genomic instability plays a crucial role in the development of small cell lung cancer (SCLC), and replication stress is a major driver of this instability. Ataxia telangiectasia and Rad3-related (ATR) are key regulators of the cellular response to replication stress, and it was found that an ATR inhibitor, berzosertib, combined with topotecan produced lasting responses in patients with chemotherapy-resistant cancers. This study investigated whether the addition of berzosertib to topotecan therapy (group 2) could provide better outcomes compared to topotecan alone (group 1) in patients with relapsed SCLC. The primary endpoint was progression-free survival (PFS), and secondary endpoints included overall survival (OS), objective response rate (ORR), and duration of response (DoR). The median PFS was 3.0 months in group 1 vs 3.9 months in group 2, with an HR 0.80 (p=.44). Median OS was 5.4 months in group 1 vs 8.9 months in group 2, with HR 0.53 (p=.03). ORR was 6% in group 1 vs 26% in group 2 (p=.15). Median DoR was 3.3 months in group 1 vs 9.3 months in group 2 (p=.45). When examined in platinum-sensitive and platinum-resistant subgroups, there were no significant differences in PFS between patients in the two treatment groups, but with OS there was a non-significant trend toward longer survival in the group 2 patients. With regards to safety, grade ≥3 treatment-related adverse events were similar between groups 1 and 2: thrombocytopenia (55% vs 50%), anemia (45% vs 45%), lymphopenia (40% vs 15%), and neutropenia (35% vs 30%). The strengths of this study included its methodology and the limitations included the small sample size. Overall, it was found that combining the ATR inhibitor berzosertib with topotecan in patients with relapsed SCLC did not significantly improve progression-free survival (PFS) compared to topotecan alone, but it significantly extended overall survival (OS) and was well-tolerated.
Click to read the study in JAMA Oncology
Relevant Reading: Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress
In-Depth [randomized controlled trial]: This open-label, phase 2 study randomized (1:2) adults with relapsed SCLC into 2 arms; topotecan alone (group 1, 20 patients) or topotecan with berzosertib (group 2, 40 patients). The median PFS was 3.0 months (95%CI, 1.2-5.1) in group 1 vs 3.9 months (95%CI, 2.8-4.6) in group 2, with an HR 0.80 (95%CI, 0.46-1.41, p=.44). Median OS was 5.4 months (95%CI, 3.2-6.8) in group 1 vs 8.9 months (95%CI, 4.8-11.4) in group 2, with HR 0.53 (95%CI, 0.29-0.96, p=.03.ORR was 6% (95%CI, 0.1%-27.3%) in group 1 vs 26% (95%CI, 13.0%-42.1%) in group 2 (p=.15). Median DoR was 3.3 months in group 1 vs 9.3 months in group 2 (p=.45). When examined in platinum-sensitive and platinum-resistant subgroups, there were no significant differences in PFS between patients in the two treatment groups, but with OS there was a non-significant trend toward longer survival in the group 2 patients. With regards to safety, grade ≥3 treatment-related adverse events were similar between groups 1 and 2: thrombocytopenia (55% vs 50%), anemia (45% vs 45%), lymphopenia (40% vs 15%), and neutropenia (35% vs 30%). Overall, it was found that combining the ATR inhibitor berzosertib with topotecan in patients with relapsed SCLC did not significantly improve progression-free survival (PFS) compared to topotecan alone, but it significantly extended overall survival (OS) and was well-tolerated.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![2 Minute Medicine: Pharma Roundup: Price Hikes, Breakthrough Approvals, Legal Showdowns, Biotech Expansion, and Europe’s Pricing Debate [May 12nd, 2025]](https://www.2minutemedicine.com/wp-content/uploads/2025/05/ChatGPT-Image-May-12-2025-at-10_22_23-AM-350x250.png)