Bevacizumab with induction and neoadjuvant chemoradiation may be effective in rectal cancer
1. In a phase II trial of T3 resectable rectal cancer, adding bevacizumab to induction and neoadjuvant chemoradiation resulted in a greater rate of pathological complete response as compared to the standard rate.
2. Toxicities included risk of postoperative fistula but were manageable; there were no treatment-related deaths.
Evidence Rating: 2 (Good)
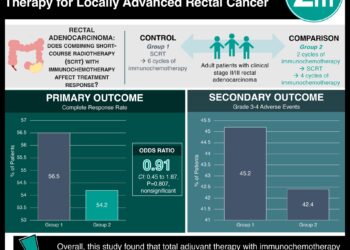
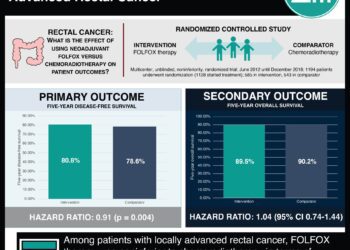
Study Rundown: Bevacizumab, or Avastin, is an antibody against the vascular endothelial growth factor A (VEGF-A) that has previously been shown to have anti-tumor effects in rectal cancer. In this phase II clinical trial, patients with MRI-defined locally advanced, T3 resectable rectal cancer were treated with bevacizumab in addition to standard regimens of neoadjuvant chemoradiation in two ways. In the first arm, 46 patients were randomized to receive a 12-week induction therapy of bevacizumab plus fluorouracil (5-FU), leucovorin, and oxaliplatin (FOLFOX-4) followed by neoadjuvant bevacizumab plus 5-FU and radiation. In the second arm, 45 patients received only 7 weeks of neoadjuvant bevacizumab plus 5-FU and radiation. All patients went on to receive a total mesorectal excision. Patients in the first arm had a complete pathological response (no evidence of disease in the surgical specimen) rate of 23.8%, which was superior to the standard rate of 10%. Patients in the second arm had a complete pathological response (pCR) rate of 11.4%, which was not significantly different from the standard rate.
In terms of safety, there was an increased risk of postoperative fistulas with the use of bevacizumab; however, this risk was similar between the two arms. There were no deaths in either treatment arm up to 8-weeks after surgery. This trial supports the use of bevacizumab as a potential induction agent in rectal cancer. However, factors that limit the generalizability of this study are the lack of direct comparisons between arms, and most importantly, no control arm consisting of the standard of care.
Click to read the study in The Annals of Oncology
Relevant reading: Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer
In-Depth [randomized controlled trial]: This study was a randomized, non-comparative, multicenter, open-label phase II trial. The inclusion criteria were patients had MRI-defined T3 resectable rectal cancer that was chemoradiotherapy-naïve. Patients were randomized to two arms, as described above. The patient populations were balanced with respect to age, performance status, histological type, tumor localization, nodal involvement, and surgery type. The analysis of these data was performed on an intention-to-treat basis and results were confirmed by local review. In the first arm (patients receiving both induction and neoadjuvant bevacizumab), the pCR rate was 23.8% (95% CI: 12.1-39.5), as compared to the standard rate of 10% (p=0.015). In the second arm (patients receiving only neoadjuvant bevacizumab), the pCR rate was 11.4% (95% CI: 3.8-24.6), as compared to the standard rate (p=0.906). During the treatment period, 50% of patients in the first arm experienced Grade 3-4 adverse events, of which 26.1% was related to bevacizumab. 20% of patients in the second arm experienced Grade 3-4 adverse events, of which 11.1% was related to bevacizumab. Adverse events during treatment included neutropenia, diarrhea, asthenia, hypertension, thromboembolism, fever, anorexia, GI perforation, and proctitis. Postoperatively, 6.5% and 13.3% of patients suffered from a fistula in the first and second arm, respectively.
More from this author: Erlotinib does not demonstrate increased survival in ovarian epithelial carcinomas, Afatinib shows increased progression-free survival in non-small-cell lung cancer, Radiosurgery alone may be effective for some arteriovenous malformations, Escalated-dose radiotherapy did not increase survival in prostate cancer, Stereotactic radiosurgery promising for patients with multiple brain metastases
Image: CC/WIki
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.