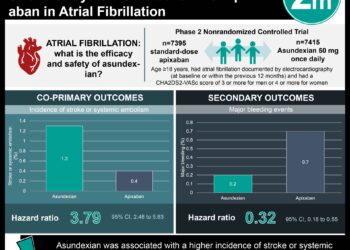
Cell-loaded scaffold implant may improve cell therapy [PreClinical]
1. A polymer scaffold modified with stimulatory molecules promoted the growth and migration of tumor-specific immune T cells in vitro and in vivo.
2. In mouse cancer models, the T cell-loaded polymer implant prevented disease relapse following surgical tumor removal and caused regression of inoperable tumors.
Evidence Rating Level: 2 (Good)
Study Rundown: Surgical removal of solid tumors can leave behind residual cancer cells that cause relapse, or in other cases such resection is not a feasible procedure. Adoptive T cell therapy, which uses infusions of anti-tumor primed immune T cells to target cancer cells, is considered an additional or alternative treatment for solid tumors. This study sought to address one of the major limitations in adoptive T cell therapy, effective delivery of activated T cells to the tumor site. Researchers developed a scaffold of alginate, a natural polymer approved by the U.S. Food and Drug Administration that degrades in the body; the scaffold was modified with collagen-like molecules and microparticles that mimicked T cell-stimulating cells. When tumor-specific T cells were loaded into the scaffold, T cell growth and migration into surrounding tissue was increased by the scaffold modifications. In a breast cancer mouse model of relapse after surgery, the polymer implant loaded with T cells improved survival and prevented tumor relapse when compared to bolus injections of the anti-tumor T cells. In an ovarian cancer mouse model of inoperable tumors, the implant improved survival and promoted tumor regression.
This study demonstrated a technology for effective T cell delivery to a specific tumor site. Compared to current methods of systemically infusing T cells, the surgical implant was more invasive, but had the advantage of improved anti-tumor activity due to localized, prolonged release of continuously stimulated large numbers of T cells. Overall, this modified, cell-loaded implant has great potential to improve adoptive T cell therapy for treating solid tumors.
Click to read the study in Nature Biotechnology
Relevant Reading: Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy
In-Depth [animal study]: The alginate macroporous scaffolds used in this study were functionalized with collagen-mimetic molecules, which promoted T cell migration, and with soluble-factor containing microparticles, intended to provide cell growth and activity signals. Because T cells respond to a variety of molecules that are soluble or attached to other cells, the microparticles were designed to release a form of the soluble factor interleukin 15 and have a lipid outer layer with bound signaling molecules. Over a seven-day period, tumor-specific T cells seeded onto the functionalized scaffold exhibited a 22-fold increase in proliferation and a 8.3-fold increase in migration out of the implant compared to cell activity in a scaffold lacking the microparticle modification.
Using a breast cancer mouse model, tumors were partially surgically removed with around 1% of residual tumor tissue left at the site. All animals that received the cell-loaded fully modified polymer implant survived for at least 80 days (last follow up) following the surgery with no tumor relapse. In contrast, animals receiving no treatment, intravenous T cell injection, or intracavitary T cell injection survived a median of 19, 21, and 25 days, respectively. In a separate experiment, a stage 3 ovarian carcinoma mouse system was used to model inoperable tumors. Tumor regression was observed only in the animals treated with the implant, with complete tumor eradication in 6 of 10 animals (at follow up of 80 days). Survival of animals with the implant was significantly higher than for animals receiving an injection of pre-activated T cells to the tumor site (p < 0.0001).
More from this author: Novel system rapidly detects low levels of bacteria in blood, Vaccines for predicted influenza strains may provide wide protection, Key gut bacteria may prevent Clostridium difficile infection, Modified embryonic stem cells may prolong survival of surgical grafts
Image: PD
©2014 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. No article should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2 Minute Medicine, Inc.