Cryoballoon ablation for prevention of atrial arrhythmia recurrence
1. Patients with recurrent atrial fibrillation who received cryoballoon ablation were significantly more likely to remain free of subsequent arrhythmia after 1 year compared to those who received antiarrhythmics.
2. Serious procedure-related events were infrequent, and the total number of serious adverse events was similar between groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: In patients with symptomatic paroxysmal atrial fibrillation, early rhythm control is associated with superior cardiovascular outcomes and may decrease progression to permanent atrial fibrillation, which is associated with irreversible morphological remodeling. Radiofrequency current ablation has consistently been shown to be more effective than antiarrhythmic drug therapy in preventing the recurrence of drug-refractory paroxysmal atrial fibrillation, but few studies have been conducted investigating whether this method is appropriate as an first-line therapy. While this intervention is associated with a relatively high incidence of severe adverse events and a relatively high rate of repeat procedures, cryoballoon ablation has emerged in recent years as an alternative, potentially safer catheterization approach. In this study, cryoballoon ablation was compared against antiarrhythmic use as an initial treatment for adult patients with recurrent symptomatic paroxysmal atrial fibrillation. At 12 months, a significantly greater percentage of patients who received cryoballoon ablation remained free of atrial arrhythmia recurrence compared to those who received drug therapy. Initial failure of the procedure occurred in under 3% of those in the ablation group, and no patients required repeat ablation. Serious adverse events related to the procedure occurred in 2% of patients in the ablation group, but the overall rate of serious adverse events was similar between groups. Longer-term and larger-scale studies are necessary to confirm the robustness and durability of treatment response, but these preliminary findings constitute additional evidence in favor of ablation as a first-line strategy for paroxysmal atrial fibrillation.
Click here to read the study in NEJM
Relevant Reading: Long-Term Outcomes After Ablation for Paroxysmal Atrial Fibrillation Using the Second-Generation Cryoballoon: Final Results from STOP AF Post-Approval Study
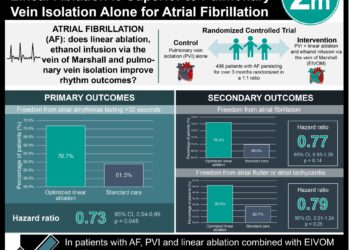
In-Depth [randomized controlled trial]: In this multicenter study with an enrollment period of June 2017 through May 2019, 225 adults who had recurrent symptomatic paroxysmal atrial fibrillation without marked left atrial enlargement were randomly assigned in a 1:1 ratio to receive either cryoballoon catheter ablation or pharmacotherapy with a class I or III antiarrhythmic drug. Patients were excluded if they had previously received any form of rhythm control. At 12 months, treatment success (defined as freedom from initial failure of the procedure or recurrence of atrial arrhythmia) was observed in 74.6% (95% CI, 65.0 to 82.0) of patients in the ablation group and 45.0% (95% CI, 34.6 to 54.7) of patients in the drug therapy group (P<0.001 by log-rank test). Notably, one-third (34%) of patients in the drug-therapy group crossed over to ablation because of drug-related side effects or recurrence of arrhythmia, and no patient in either group underwent ablation more than once. Within the per-protocol ablation group, two patients (1.9%; 95% CI, 0.5 to 7.5) suffered from a serious procedure-related event (pericardial effusion or myocardial infarction); one patient who received ablation after crossing over also had a major vascular complication. However, no between-group difference was observed in the total occurrence of serious adverse events. Ablation was also associated with dramatic quality-of-life improvements as measured by both the AFEQT and the EQ-5D, but the two groups were similar with respect to subsequent cardiovascular-related health care utilization (69.9% vs. 53.5%) or free from cardioversion (97.1% vs. 92.4%).
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.