Dabigatran linked to fewer major bleeding events than warfarin in ablation: The RE-CIRCUIT trial
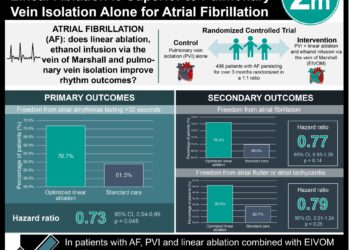
1. The incidence of major bleeding events up to 8 weeks after catheter-directed atrial fibrillation ablation was significantly lower in those receiving continuous therapy with dabigatran compared to those receiving INR-directed warfarin.
2. Both groups experienced similar incidence of minor bleeding events and thromboembolic events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Catheter-directed ablation therapy of paroxysmal and persistent atrial fibrillation is a relatively common procedure, with the most feared potential complication being stroke. Prior research has linked uninterrupted therapy with warfarin during the time of ablation with a lower risk of periprocedural bleeding and stroke compared to stopping warfarin and bridging with low-molecular-weight heparin. However, there are gaps in our understanding regarding the relative risks and benefits of uninterrupted non-vitamin K antagonist oral anticoagulant therapy around the time of ablation.
The RE-CIRCUIT trial involved 635 patients who underwent catheter ablation for atrial fibrillation, randomizing them to receive 4 to 8 weeks of uninterrupted anticoagulation with either warfarin or dabigatran. The incidence of major bleeding events up to 8 weeks after ablation was significantly lower in the dabigatran group compared to the warfarin group, and both groups experienced similar incidence of minor bleeding events and thromboembolic events. These findings advance the possibility that continuous therapy with non-vitamin K antagonist oral anticoagulant therapy during ablation could be a superior approach in the future. The trial is limited somewhat by its open label design, and also by the fact that there was only 66% adherence (measured by INR in the 2.0 to 3.0 range) in the warfarin group compared to mean adherence of 97.6% in the dabigatran group. Furthermore, while a specific dabigatran-reversal agent became available during the course of the trial, it was not utilized for the purposes of this study, and future research will necessarily incorporate this antidote into the treatment protocol. Of note, the study was funded by the producer of dabigatran.
Click to read the study, published in NEJM
Relevant Reading: Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation
In-Depth [randomized controlled trial]: This randomized, open-label, multicenter, controlled trial involved 704 patients from 104 sites, 635 of whom underwent catheter ablation for paroxysmal or persistent atrial fibrillation. Patients were well matched and were randomized to receive either warfarin, titrated to achieve an INR goal of 2.0 to 3.0, or dabigatran at a dose of 150 mg twice daily, for a pre-ablation phase of 4 to 8 weeks, after which they underwent catheter ablation. The primary end point was the incidence of adjudicated major bleeding events, such as pericardial tamponade or effusion, intracranial hemorrhage, or retroperitoneal bleeding during the 8 weeks following the procedure. This occurred in significantly fewer patients in the dabigatran group compared to the warfarin group (5 patients, 1.6% vs. 22 patients, 6.9%; absolute risk difference of -5.3 percentage points 95%CI -8.4 to -2.2; p < 0.001), amounting to a relative risk reduction in favor of dabigatran of 77.2. This corresponded to an early and persistent separation of curves in the Kaplan-Meier plot over the course of follow-up. There were no strokes, embolic phenomena, or TIAs in the dabigatran group, compared to one in the warfarin group from the time of the ablation to the 8 week mark. There were similar rates of minor bleeding events in the two groups. Severe adverse events, defined as untoward medical occurrences in patients receiving a trial drug and which was incapacitating or caused an inability to work, occurred in 3.3% and 6.2% of the dabigatran and warfarin groups, respectively.
Image: PD
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.