Daily citalopram associated with decreased agitation in Alzheimer’s disease
Image: PD
1. Daily treatment with citalopram in patients with probable Alzheimer disease was associated with decreased agitation compared to placebo.
2. Citalopram use in Alzheimer’s disease patients is associated with QTc interval prolongation and worse Mini-Mental Status Exam scores.
Evidence Rating Level: 2 (Good)
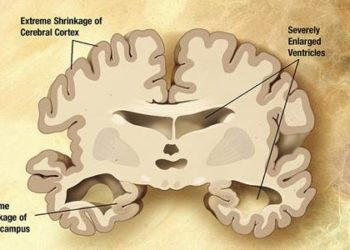
Study Rundown: Previous research has suggested that citalopram, a selective serotonin reuptake inhibitor, may be a potential substitute for antipsychotic drugs when treating agitation in patients with Alzheimer’s disease (AD). This study (Citalopram for Agitation in Alzheimer Disease Study, CitAD) aimed to determine the effect of citalopram on patients’ functional abilities and elucidate any safety concerns. The study results suggest that treatment with citalopram significantly decreased agitation on two standardized scales. It should be noted, however, that citalopram use was also associated with QTc interval prolongation and significantly lower Mini-Mental Status Exam (MMSE) scores. This study is important for identifying a potentially clinically relevant treatment effect using citalopram, but its use in everyday practice may be limited by safety concerns.
Click to read the study, published today in JAMA
Click to read the accompanying editorial in JAMA
Relevant Reading: Management of agitation and aggression associated with Alzheimer disease
In-Depth [randomized controlled trial]: This multi-center, double-blind, placebo-controlled trial randomized 186 patients with probable AD (defined by the National Institute of Neurological and Communication Disorders and Stroke-Alzheimer Disease and Related Disorders Association criteria) to receive either nine weeks of citalopram or placebo. At 9 weeks, these participants had significantly lower scores on the NBRS-A scale (Neurobehavorial Rating Scale-Agitation, mean -0.93, 95%CI -1.80 to -0.06,p=0.04). In addition, 40% of treated participants had much or very much improved mADCS-CGIC (modified Alzheimer Disease Cooperative Study-Clinical Global Impression of Change) scores compared to 26% of participants in the placebo group (OR 2.13, 95%CI 1.23-3.69, p=0.007). However, participants using citalopram were also significantly more likely to have lower MMSE (Mini-Mental Status Exam) scores (p=0.03), report diarrhea (p=0.03), and have an increased QTc interval (p=0.004).
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.