Dapagliflozin associated with reduced hospitalization rate for heart failure
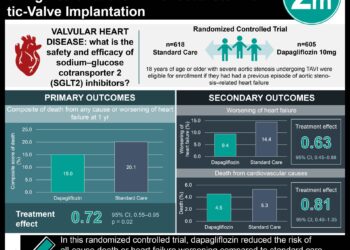
1. Among patients with type 2 diabetes and established or multiple risk factors for cardiovascular disease (CVD), dapagliflozin was noninferior but did not significantly reduce the rate of major adverse cardiovascular events (MACE) compared to placebo.
2. Dapagliflozin significantly reduced the hospitalization rates for heart failure compared to placebo in both patients with established CVD and those with only multiple risk factors.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Two sodium-glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin, have been shown to decrease cardiovascular morbidity and mortality in type 2 diabetic patients at high risk for CVD. This reports the results of the DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58) trial, designed to evaluate the cardiovascular safety and efficacy of the SGLT2 inhibitor dapagliflozin. Over 17,000 patients with type 2 diabetes and established or multiple risk factors for CVD were randomized to dapagliflozin or placebo. The investigators found that dapagliflozin was noninferior but not superior compared to placebo with respect to the incidence of MACE (the primary outcome). Dapagliflozin significantly reduced the composite of cardiovascular death and hospitalization for heart failure; however, this was due to a significant reduction in hospitalizations for heart failure without a significant difference in cardiovascular death. This trial suggests dapagliflozin is has a safe cardiovascular profile in patients with type 2 diabetes, and also suggests that the heart failure benefit of SGLT2 inhibitors extends to the primary prevention population.
This was a large, randomized trial that added to the growing body of safety and efficacy data for the SGLT2 inhibitors. Another major strength was the large primary prevention population included in the study (nearly 60% of participants), which generalize the results to a broader range of type 2 diabetes patients. A limitation of this study includes the potential for higher medication compliance than seen in the general diabetic population, given the one-month trial run-in period.
Click to read the study in NEJM
Relevant Reading: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes
In-Depth [randomized controlled trial]: Patients (17,160) with established or multiple risk factors for CVD (40.7% with established CVD) were randomized to treatment with dapagliflozin (n=8,582) or a matching placebo (n=8,578). The primary safety outcome was incidence of MACE (defined as a composite of cardiovascular death, myocardial infarction, or ischemic stroke), and the two primary efficacy outcomes were MACE and a composite of cardiovascular death or hospitalization for heart failure. Prespecified secondary outcomes included a renal composite outcome (sustained decrease in estimated glomerular filtration rate [eGFR], new end-stage renal disease, or death from renal or cardiovascular causes) and death from any cause. Serious adverse events were collected throughout the trial period.
Median follow-up duration was 4.2 years. The average reduction in hemoglobin A1C with dapagliflozin was 0.42%. The primary outcome of MACE for dapagliflozin vs. placebo met the prespecified threshold for noninferiority (upper boundary of the 95% confidence interval [CI], <1.3; P<0.001), but not superiority (8.8% vs. 9.4%, respectively; hazard ratio [HR], 0.93; 95% CI, 0.84 to 1.03; P=0.17). There was a significant difference in the composite of cardiovascular death and hospitalization for heart failure (4.9% vs. 5.8%; P=0.005); however, this was due to a significantly lower rate of hospitalization for heart failure (2.5% vs. 3.3%; P<0.005) without a significant difference in cardiovascular death (2.9% vs. 2.9%; HR, 0.98; 95% CI, 0.82 to 1.17). Among secondary outcomes, there was a significant difference in composite renal outcome (4.3% vs. 5.6%; HR, 0.76; 95% CI, 0.67 to 0.87), and no significant difference in all-cause mortality (9.2% vs. 6.6%; HR, 0.93; 95% CI, 0.82 to 1.04). The results regarding cardiovascular death and hospitalization for heart failure were similar across subgroups (including established CVD and those with only risk factors). Among additional safety outcomes, the dapagliflozin group had a higher rate of diabetic ketoacidosis (0.3% vs. 0.1%; P=0.02) and genital infections (0.9% vs. 0.1%; P<0.001). There was no significant difference in the rate of amputations, stroke, or fractures between dapagliflozin and placebo.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc