Denosumab may increase risk of major adverse cardiovascular events but reduce fracture risk in patients receiving dialysis
1. In this target trial emulation study, denosumab was associated with an increased risk of major adverse cardiovascular events compared with oral bisphosphonates among patients with osteoporosis who were also receiving dialysis.
2. However, denosumab was also found to be associated with a lower risk of composite fractures versus bisphosphonates.
Evidence Rating Level: 2 (Good)
Study Rundown: The management of osteoporosis remains challenging in patients with severe chronic kidney disease, particularly those undergoing dialysis. The monoclonal antibody denosumab is not excreted via the kidneys and is thus not affected by renal impairment, making it an enticing treatment option among dialysis-dependent patients with osteoporosis. However, a previous population-based cohort study has suggested that denosumab may be associated with severe hypocalcemia versus oral bisphosphonates, another popular class for the treatment of osteoporosis. Thus, this study aimed to compare denosumab and oral bisphosphonates with regard to the risk of major adverse cardiac events (MACE) and fracture prevention among patients receiving dialysis with comorbid osteoporosis. It was found that denosumab users had a higher risk of developing MACE over a 3-year period compared with bisphosphonate users. However, denosumab users also had a lower risk for composite fractures compared with bisphosphonate users. Both effects were more pronounced among participants who were in the study for longer than 18 months. The generalizability of this study was limited by lack of data on disease severity, the potential for outcome misclassification, and a relatively homogenous study population. Nevertheless, this study contributed to the literature surrounding the effectiveness and safety of denosumab compared with bisphosphonates.
Click to read the study in AIM
Relevant Reading: Severe Hypocalcemia With Denosumab Among Older Female Dialysis-Dependent Patients
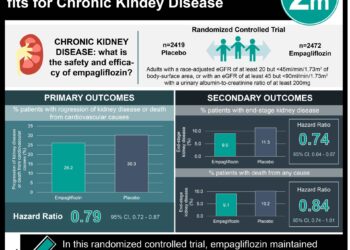
In-Depth [retrospective cohort]: This study investigated the safety and effectiveness of denosumab compared with oral bisphosphonates among Japanese patients with osteoporosis who were also receiving dialysis. Patients were identified on a claims database covering January 2014 through October 2022. Exclusion criteria included patients who had undergone dialysis for fewer than 90 days before start of follow-up; diagnosis of Paget’s disease of bone, a malignant tumor, or a giant cell tumor; history of renal transplant; use of denosumab or bisphosphonates before start of follow-up; and hospitalization for acute myocardial infarction, stroke, or heart failure within 90 days before start of follow-up. Safety was measured using MACE, which comprised myocardial infarction, stroke, hospitalization for heart failure, and cardiovascular death. The study identified a final cohort of 658 denosumab users and 374 bisphosphonate users, with an average age of 74.5 years and 62.9% being women. During the 3 years of follow-up, 146 out of 658 denosumab users developed MACE, yielding a 3-year risk of 34.6%; by contrast, 54 out of 374 bisphosphonate users developed MACE. The weighted 3-year risk difference was 8.2% (95% CI, -0.2% to 16.7%), while the weighted 3-year risk ratio was 1.36 (95% CI, 0.99 to 1.87). In the denosumab group, 25 out of 658 participants developed composite fractures; 29 out of 374 bisphosphonate users developed composite fractures. The weighted 3-year risk difference was 5.3% (95% CI, -11.3% to -0.6%), while the weighted 3-year risk ratio was 0.55 (95% CI, 0.28 to 0.93). Among patients who were in the study for over 18 months, the weighted 3-year risk difference was 14.9% (95% CI, 0.9% to 28.8%) for MACE and -9.9% (95% CI, -20.8% to -1.6%) for composite fractures. Overall, this study showed that denosumab may increase the risk of MACE while also reducing the risk of fractures.
Image: PD
©2025 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.