Differential response to panitumumab seen in colorectal cancer based on genotypes
Image: PD
1. Addition of panitumumab to the first-line FOLFOX4 regimen prolonged survival in patients without RAS mutations.
2. Panitumumab was associated with shortened survival in patients with certain RAS mutations.
Evidence Rating Level: 1 (Excellent)
Study Rundown: This study demonstrated the genetic heterogeneity of colorectal cancer and the resultant complications in finding the optimal treatment regimen. Adding panitumumab to the established first-line regimen of oxaliplatin, fluorouracil and leucovorin (FOLFOX4) was associated with better survival outcomes in some tumor genotypes and worse outcomes in others. As such, selection of the optimal treatment should take into account the specific genetic markers of the tumor, among other considerations. This conclusion reasonably applies to other types of cancer where high genetic heterogeneity is common. The study is strong with a large sample size and is well-controlled for subgroup differences. Amgen is the study sponsor and the manufacturer for panitumumab.
Click to read the study, published today in NEJM
Click to read an accompanying editorial in NEJM
Relevant Reading: Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer
In-Depth [prospective-retrospective analysis]: This study analyzed the survival and progression benefits of adding panitumumab to oxaliplatin, fluorouracil and leucovorin (FOLFOX4) as it related to tumor genotypes.
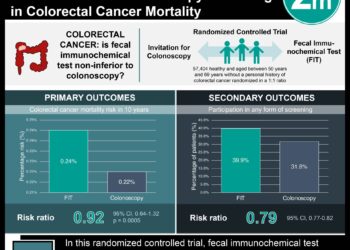
In patients without RAS mutation, the panitumumab-FOLFOX4 group had better progression-free survival (10.1 vs 7.9 months, P=0.004) as well as better overall-survival (26.0 vs 20.2 months, P=0.04) compared to the FOLFOX4 group. In patients without KRAS mutation in exon 2, the panitumumab-FOLFOX4 group had better progression-free survival compared to the FOLFOX4 group (9.6 vs 8.0 months, P=0.02). Overall survival also favored addition of panitumumab but was not statistically significant (23.9 vs 19.7 months, P=0.07).
In patients without KRAS exon 2 mutation but with other RAS exon mutations, progression-free survival and overall survival were shorter with addition of panitumumab, though this was not statistically significant. Panitumumab-FOLFOX4 group had inferior progression-free survival in patients with KRAS mutations in exon 2 (7.3 vs 8.8 months, P=0.02). As such, the survival benefit of adding panitumumab to the FOLFOX4 regimen was not consistent across tumour genotypes.
By Xiaozhou Liu and Adrienne Cheung
More from this author: Nivolumab plus ipilimumab shows promise for metastatic melanoma, Prophylactic platelet transfusions prevent bleeding in hematologic cancers, Azithromycin is not associated with increased cardiovascular death in low-risk groups, Health education module reduces parasitic infections, New chemotherapy precludes the need for radiotherapy in primary mediastinal B-cell lymphoma.
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.