Direct oral anticoagulants have similar bleeding risk compared with aspirin
1. In this systematic review and meta-analysis, the non-vitamin K oral anticoagulants apixaban and dabigatran both had similar rates of major bleeding and intracranial hemorrhage compared with aspirin monotherapy.
2. Rivaroxaban was found to have numerically greater rates of major bleeding and intracranial hemorrhage compared with aspirin, though this difference was not statistically significant.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Although non-vitamin K oral anticoagulants (NOACs) have been shown to be effective for stroke prophylaxis in patients with atrial fibrillation (AF) and treatment of venous thromboembolism, their risk of bleeding compared with antiplatelet monotherapy remains unclear. This study aimed to synthesize the literature comparing bleeding risk, particularly major bleeding and intracranial hemorrhage, between NOACs and aspirin. It was found that apixaban and dabigatran were associated with similar rates of major bleeding compared with aspirin. Rivaroxaban was noted to be associated with a higher absolute risk of major bleeding, but this difference was not statistically significant. Apixaban and dabigatran were also found to have similar rates of intracranial hemorrhage compared with aspirin, while rivaroxaban again had a higher rate of intracranial hemorrhage that was not statistically significant. The generalizability of this study was limited by the imprecision of the results, as well as a lack of comparisons between other NOACs and other antiplatelet medications. Nevertheless, this study showed that NOACs generally had similar rates of bleeding events compared with aspirin monotherapy, which may inform treatment decisions for patients in need of anticoagulation.
Click to read this study in AIM
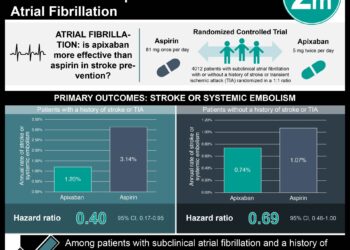
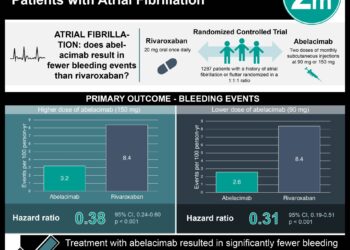
In-Depth [systematic review and meta-analysis]: This systematic review and meta-analysis investigated risk of bleeding between NOACs and antiplatelet monotherapy. Databases were searched for randomized controlled trials (RCTs) comparing therapeutic-dose NOACs with antiplatelet monotherapy for at least 3 months. A total of 9 RCTs were included, yielding a participant population of 26,224. Of these trials, 6 were judged to be at low risk of bias and 3 were at high risk of bias. Apixaban, dabigatran, and rivaroxaban were the NOACs that were studied, while the only antiplatelet used was aspirin. A total of 566 patients (2.16%) had major bleeding. Compared to aspirin, apixaban (risk difference [RD], 0.0 percentage points [95% CI, -1.3 to 2.6]) and dabigatran (RD, 0.5 percentage points [95% CI, -2.1 to 19.6]) had similar major bleeding rates, while rivaroxaban had higher rates (RD, 0.9 percentage points [95% CI, -0.1 to 3.7]). Similarly, the rates of intracranial hemorrhage were slightly lower compared to aspirin for apixaban (RD, -0.2 percentage points [CI, -0.6 to 1.4]) and dabigatran (RD, 0.0 percentage point [CI, -1.1 to 24.5 percentage points]), and slightly higher for rivaroxaban (RD, 0.3 percentage point [CI, -0.1 to 79.7 percentage points]). Overall, this study indicated that apixaban and dabigatran have similar bleeding risk compared with aspirin monotherapy, while rivaroxaban may confer a greater risk.
Image: PD
©2025 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.