Discontinuing oxytocin during active labour has no significant impact on neonatal morbidity
1. Neonatal morbidity rate was comparable between the continuous oxytocin and discontinuous oxytocin groups.
2. There was no significant difference in adverse events between both groups.
Evidence Rating Level: 1 (Excellent)
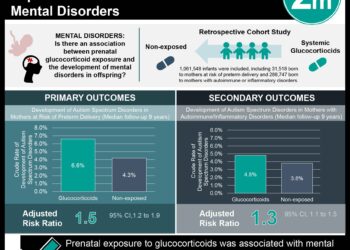
Study Rundown: Oxytocin is known for reducing labour duration but may pose risks for neonatal morbidity. In the multicenter STOPOXY trial, participants receiving oxytocin in early labour were randomly assigned to discontinuous or continuous administration. This randomized controlled trial aimed to assess the impact of discontinuing oxytocin during active labour on neonatal morbidity rate. The primary outcome was neonatal morbidity, assessed through a composite of umbilical arterial pH, lactate, base excess, APGAR score, and neonatal intensive care unit admission, while key secondary outcomes were maternal and fetal complications. According to study results, there was no significant difference in neonatal morbidity between the continuous and discontinuous oxytocin groups. Although well-executed, a major limitation of this study was the lack of assessment for long-term effects on neonatal health.
Click to read the study in The Lancet
Relevant Reading: Heat-Stable Carbetocin versus Oxytocin to Prevent Hemorrhage after Vaginal Birth
In-depth [randomized-controlled trial]: Between Jan 13, 2020, and Jan 24, 2022, 2459 patients were screened for eligibility across 21 maternity units in France. Included were patients receiving oxytocin before 4 cm dilation, randomly assigned 1:1 to discontinuous or continuous oxytocin in the multicenter STOPOXY trial. Altogether, 2170 patients (1067 in discontinuous oxytocin and 1103 in continuous oxytocin) were included in the final analysis. The primary outcome of neonatal morbidity was comparable in the discontinuous oxytocin (9.6%, 95% confidence interval [CI] 7.9-11.5) and continuous oxytocin (9.2%, 95% CI 7.6-11.0) groups, with no significant difference observed (relative risk [RR] 1.0, 95% CI 0.8-1.4). The secondary outcomes revealed no clinically significant differences in adverse events between the two groups. Findings from this study suggest that discontinuing oxytocin when reaching the active phase in early labour does not significantly impact neonatal morbidity compared to continuous administration.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.