Donepezil and vitamin E in Alzheimer’s disease [Classics Series]
Image: PD
1. High-dose vitamin E supplementation does not slow down progression to Alzheimer’s disease
2. Donepezil, a cholinesterase inhibitor, shows modest reductions in risk of progression to Alzheimer’s disease early in treatment
Original Date of Publication: June 9, 2005
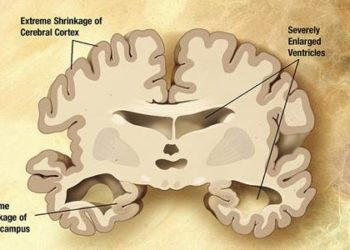
Study Rundown: Around 80% of people who meet criteria for amnestic mild cognitive impairment go on to develop Alzheimer’s disease (AD) within the next six years. There has been a lot of research focused on ways of slowing down the process of cognitive impairment, including medications such as donezepil and supplements such as vitamin E. This study compared donepezil (a cholinesterase inhibitor), high-dose vitamin E supplementation, and placebo with regards to their effect at slowing down the progression to AD. The study found that while donepezil has some modest effects early on in treatment, vitamin E was not superior to placebo.
In summary, this study showed the modest effects of donezepil at reducing the progression to AD and that vitamin E was not effective in slowing down the progression of mild cognitive impairment. As shown in previous studies, this study also demonstrated that being a carrier of the APO-E e4 allele is the most significant risk factor in developing AD. Given the allele’s propensity for AD, it was included as a covariate when running statistical analysis of data. However, the paper did not show any data concerning the covariate.
Click to read the study in NEJM
Study Author, Dr. Ronald C. Petersen MD PhD, talks to 2 Minute Medicine: Director of Alzheimer’s Disease Research Center, Mayo Clinic College of Medicine
“While the overall outcome of the study was negative, it raised the issue of using cholinesterase inhibitors in some subsets [of] patients with MCI highlighting the concept of biomarker stratification. That is, subjects who were carriers of the apolipoprotein E4 allele may have responded differently than the overall group of MCI subjects suggesting that certain subsets of subjects might be more appropriate for particular therapies.
Many studies are under way or being planned stratifying subjects with MCI according to their underlying biomarker profiles. This will likely enhance the probability of finding specific treatments for subsets of patients based on their biomarker profiles.”
In-Depth [randomized, controlled study]: This multicenter, randomized, double-blind, placebo-controlled trial took 769 subjects with mild cognitive impairment from across the United States and Canada and randomized them to receive either 2000 IU of vitamin E with a placebo, 10 mg of donezepil with a placebo, or placebos for both. All patients also took a daily multivitamin. Subjects were screened for mild cognitive impairment by several independent measures and were all between 55-90 years of age. The primary end point was time to the development of possible or probable Alzheimer’s disease, which was defined according to clinical criteria by multiple independent national neurological and Alzheimer’s disease organizations. Secondary outcomes included scores on a variety of assessment scales testing different aspects of cognition. Patients were followed for 3 years.
Results showed that there was no significant difference in progression to AD between the vitamin E group and the placebo group at any time during the 3 year trial (numerical data not provided in paper). Donezepil, on the other hand, did show modest reduction in risk of progression to AD compared to placebo, for the first 12 months of the trial (p=0.004 at 6 months and p=0.04 at 12 months). During years 2 and 3, the hazard ratios were lower, but still significant (p=0.03 for both years). However, by 36 months, the three groups did not differ significantly in the number of subjects who had progressed to AD (63 in donezepil group vs. 73 in placebo group, p=0.21). Secondary outcomes showed minor improvements in some of the assessments in the donezepil group compared to the placebo group but the differences were confined to the first 18 months of the study. The one marker that stood out was the APO-E e4 allele, with 76% of AD cases in the study occurring among carriers of the allele (p<0.001).
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.