Endovascular stroke treatment reduces mortality
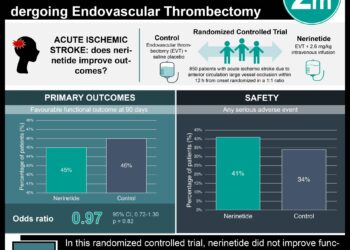
1. Patients with acute ischemic stroke presenting with proximal vessel occlusion, adequate collateral circulation, and small infarct size who received rapid endovascular treatment had significantly improved 90-day functional outcomes and reduced mortality when compared to those who received intravenous alteplase alone.
Evidence Rating: 1 (Excellent)
Study Rundown: Patients with acute ischemic stroke with a proximal vessel occlusion of the anterior circulation fare poorly despite the current standard-of-care. A significant percentage of these patients die or do not regain functional independence at 90 days. Given the recent publication of the MR CLEAN, this study, the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE), sought to further define the beneficial outcomes associated with endovascular therapy for acute ischemic stroke. The ESCAPE investigators randomized patients with acute ischemic stroke from proximal occlusion of an anterior circulation artery to receive rapid endovascular treatment and compared outcomes to those who underwent standard therapy with intravenous alteplase. The results demonstrated significantly improved functional and mortality outcomes at 90 days among those within the trial’s endovascular arm. This trial was stopped early due to its efficacy, and is the first trial to show a mortality benefit for endovascular stroke treatment. In addition to being a multi-site randomized controlled trial, this study increased its generalizability by including patients of all ages and allowing enrollment time up to 12 hours from first symptom onset. However, this study was performed at sites with adequate infrastructure to provide efficient and rapid delivery of imaging and reperfusion therapy, which is not currently widely available. Also, there is no mention of how many people were excluded from the study, nor an indication of what percentage of all ischemic stroke patients would benefit from this type of intervention. Future studies should try to broaden the inclusion criteria and determine whether other types of ischemic strokes with different vascular patterns would benefit from an endovascular strategy.
Click here to read the study in the New England Journal of Medicine
Relevant reading: A randomized trial of intraarterial treatment for acute ischemic stroke
In-Depth [randomized controlled trial]: A total of 316 stroke patients around 22 centers worldwide who presented with proximal vessel occlusion in the anterior circulation, small infarct cores, and adequate collateral circulation were enrolled in the study. Eligible patients were adults of any age who were functionally independent prior to their stroke. All patient within the endovascular arm of the trial received treatment within 12 hours of symptom onset. Both the intervention arm and control arm received current standard-of-care alteplase treatment, when appropriate. Within the endovascular therapy arm, the majority of patients were treated with retrievable stents (86.1%). The goal time from imaging to the beginning of the procedure was set as 60 minutes, and a goal of 30 minutes for set for successful reperfusion; patients in whom these goals could not be reasonably met were excluded from enrollment. The study’s primary outcome measure was the modified Rankin scale score (mRSS) at 90 days. Prior to full enrollment, the study was stopped due to evidence of treatment efficacy. The 165 patients that underwent rapid endovascular treatment had better rates of functional independence (90-day mRSS 0-2) when compared to the 150 patients in the control group (53% vs. 29.3%, P<0.001). The primary outcome favored the intervention arm (common odds ratio, 2.6; 95% CI, 1.7-3.8, P<0.001). Additionally, the intervention group had reduced mortality (10.4% vs. 19.0%, P=0.04), and there was no significant difference in symptomatic intracerebral hemorrhage (3.6% intervention, 2.7% control, P=0.75).
Image: CC/Martin Hasselblatt MD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.