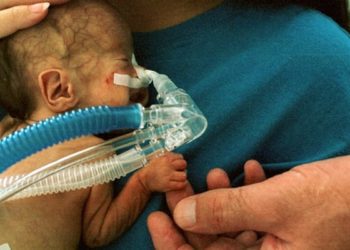
Erythropoietin prophylaxis unlikely to improve neurodevelopment in very preterm infants
1. Very preterm infants who were given high dose recombinant human erythropoietin (rhEPO) after birth did not show improved neurodevelopmental outcomes at two years of follow-up.
2. Survival without severe neurodevelopmental impairment was also not improved in the rhEPO group compared to the placebo controls.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Infants born at 26 to 32 weeks of age (very early preterm) demonstrate poorer neurodevelopmental outcomes compared to those born later during the gestational period. Previous studies have shown that recombinant human erythropoietin (rhEPO) may be neuroprotective when administered at high doses prophylactically after birth. In this randomized controlled trial, two groups of very preterm infants received either three doses of rhEPO or placebo within a 42 hour period after birth. At two years of follow up, infants who received rhEPO did not demonstrate better outcomes on a neurodevelopmental assessment index compared to placebo. Secondary outcomes and exploratory analyses, including measures of psychomotor function, mean body weight and length, head circumference, and severe visual or hearing impairment, were also not significantly different between groups. Both groups showed similar rates of survival without neurodevelopmental impairments.
These results do not lend support for the theory that rhEPO is beneficial for neurodevelopmental outcomes in very preterm infants. However, it is not clear what effects early rhEPO prophylaxis might have at later time points or whether more prolonged doses of rhEPO might be beneficial. Other ongoing clinical trials utilizing different dosing regimens and measuring outcomes at different time points may reveal more effective strategies for rhEPO prophylaxis in very preterm infants.
Click to read the study, published today in JAMA
Relevant Reading: Association Between Early Administration of High-Dose Erythropoietin in Preterm Infants and Brain MRI Abnormality at Term-Equivalent Age
In-Depth [randomized controlled trial]: A total of 450 very preterm infants (born between 26 weeks 0 days and 31 weeks 6 days of gestation) were recruited across 5 Swiss hospitals to receive either 3000 IU/kg rhEPO or placebo in 3 doses at 3 hours, 12 – 18 hours, and 36 – 42 hours after birth. Infants with severe congenital malformations, genetically defined syndromes, severe intraventricular hemorrhage, or a prior palliative care consult were excluded. At two years of follow up, the rhEPO (83% follow-up) and placebo (79% follow-up) groups were given neurodevelopmental examinations using the Bayley Scales of Infant Development-II (BSID-II), Mental Development Index (MDI), and Psychomotor Development Index (PDI), among others. The rhEPO group did not show a statistically significant difference on the MDI (Difference: -1; CI95 -4.5 to 2.5) or PDI (Difference: -2.6; CI99 -7.7 to 1.7) compared to the placebo group. In addition, there were no statistically significant differences in the rate of neurodevelopmental disorders, visual or hearing impairment, or measures of weight, length, and head circumference. The rate of survival without neurodevelopmental impairment was also similar between the two groups (OR = 0.9; CI99 0.4 to 1.9).
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.