GnRH agonist for ovarian preservation in breast cancer (POEMS Study)
1. Women treated with a gonadotropin-releasing hormone (GnRH) agonist were less likely to undergo ovarian failure during chemotherapy.
2. Women who received a GnRH agonist were more likely to subsequently become pregnant.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Treatments for breast cancer have advanced considerably over the past few decades. While chemotherapy can be life-saving, as chemotherapeutic agents target rapidly dividing cells, damage often occurs to not only breast cancer cells, but also rapidly dividing ovarian cells. Resulting infertility and premature menopause can be emotionally stressful and result in associated health problems including decreased bone density.
Goserelin, or Zoladex, is an FDA-approved gonadotropin releasing hormone (GnRH) agonist used to treat prostate cancer, certain breast cancers, and benign gynecologic diseases. By inhibiting the hormones that control ovulation, goserelin shuts down the hypothalamic-pituitary-ovarian axis, potentially exposing ovaries to less risk from the toxic effects of chemotherapy. However, studies testing this hypothesis have yielded mixed results. In this international, phase 3, randomized study, researchers measured the effect of goserelin treatment during chemotherapy on rates of ovarian failure and successful pregnancy in premenopausal women with hormone-receptor negative breast cancer.
Women randomized to receive goserelin were less likely to experience ovarian failure and more likely to become pregnant. Strengths included relatively long follow-up time (2 years for ovarian reserve and a median of 4 years for secondary outcomes like pregnancy). A significant limitation was the large number of patients with missing data—only 135 of the 218 eligible participants had enough information available to evaluate ovarian reserve. Although sensitivity analyses suggest that the missing data did not significantly affect the results, larger studies with better retention are merited.
Click to read the study in NEJM
Relevant Reading: Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage
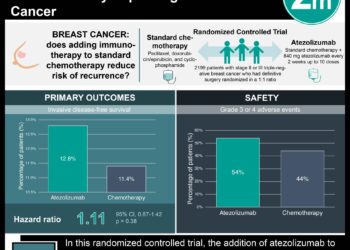
In-Depth [randomized controlled trial]: Premenopausal women with operable hormone-receptor-negative breast cancer were randomized to receive both standard chemotherapy and the GnRH agonist goserelin (n = 126) or standard chemotherapy alone (control) (n = 131). The primary outcome was ovarian failure at 2 years of follow-up, defined as the absence of menses for 6 months and elevated FSH levels in the postmenopausal range. Secondary endpoints were pregnancy outcomes and disease-free and overall survival.
Women randomized to receive a GnRH agonist were less likely to experience ovarian failure than controls (8% vs. 22%, OR 0.30, p = 0.04). Pregnancy was more common among women in the goserelin group than in the control group (21% vs. 11%, p = 0.03), although a greater proportion of women in the GnRH agonist group attempted pregnancy compared with those in the control group (24% vs. 16%). Women in the GnRH agonist group also experienced longer disease-free survival and overall survival compared to controls.
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.