Human neural stem cell grafts in rhesus monkeys improve motor function after spinal cord injury [PreClinical]
1. Following cervical spinal cord injury, rhesus monkeys were grafted with human neural progenitor cells.
2. Over the course of 9 months, grafted human neural progenitor cells matured and were found to improve forelimb function in rhesus monkeys.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Recovery after spinal cord injury (SCI) has proven challenging, largely due to the inability of axons to regenerate. Neuronal and axonal regeneration can be promoted through neural stem cell grafts. In order to be successful, grafted neurons must be able to survive and mature in their new environment, extend axons into the spinal cord, and synapse appropriately. Researchers had previously optimized methods for grafting neural stem cells into rodents following SCI. In this study, improved methods were used to graft neural stem cells into rhesus monkey models of SCI.
Nine adult male rhesus monkeys received right-sided C7 hemisection spinal cord lesions. Two weeks later, at the site of the spinal cord lesions, monkeys were grafted with 20 million GFP-expressing human neural progenitor cells. These neural progenitor cells had been isolated from an 8-week-old human embryonic spinal cord and maintained in vitro as a stable multipotent cell line. After grafting failed in initial trials, methods to graft neural cells in monkeys were further optimized. Draining of the cervical spinal cord of cerebrospinal fluid, increasing fibrin-thrombin concentration into the grafting mixture, and increasing the dose of immunosuppressive medications, were measures that improved grafting.
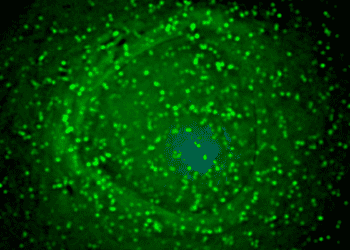
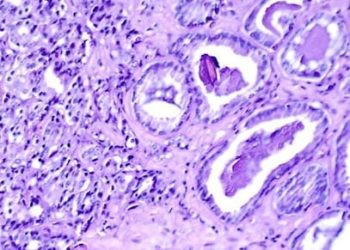
Successful grafts tended to occupy the majority of the lesion cavity and generally integrated well with the host spinal cord. About 60% of grafted cells expressed neuronal markers, and 40% expressed glial cell (astrocyte and oligodendrocyte) markers. Human axons emerged from the grafts in large numbers. Additionally, these axons extended long distances – up to 50mm – away from the graft site. In a task that assessed over 25 features of motor function, monkeys with surviving grafts had superior performance measures compared to monkeys with non-surviving grafts.
One major barrier to the axon regeneration field has been the inhibitory nature of myelin to axon growth. In this study, the authors demonstrate that human neural progenitor cell grafts permit axonal growth through white matter. Furthermore, graft methods developed in primate models can inform future clinical trials. Though questions regarding graft maturity, delayed effects, and functional outcomes remain, this pre-clinical model suggests that neural stem cell engraftment in humans following spinal cord injury may represent a viable therapeutic strategy.
Click here to read the study in Nature Medicine
Relevant Reading: Indications and prospects of neural transplantation for chronic neurological diseases
In-Depth [animal study]: Nine adult male rhesus monkeys received right-sided C7 hemisection lesions. Two weeks later, cerebrospinal fluid was drained from the cervical spinal cord and 20 million GFP-expressing human neural stem cells were grafted into the lesion cavity, suspended in a medium containing growth factors and fibrin-thrombin matrix. A robust immunosuppressive regimen, consisting of myophenolate mofetil and tacrolimus, was necessary to prevent graft rejection.
The animals were followed for up to 9 months postgrafting. Tissue sections were obtained periodically to evaluate neuronal marker expression near the graft sites. The relative proportion of cells expressing CNS markers in grafted cells was quantified – 57% of cells were neuronal (NeuN+), 26% were astrocytes (SOX9+), and 17% were oligodendrocytes (OLIG2+). To examine axonal migration distances, an anterograde neuronal tracer was injected into areas surrounding the graft. Up to 150,000 axons were detected 2mm caudal to the lesion 2-9 months postgrafting, and extended through white matter. Delayed expression of mature neuronal markers (NF200) indicated that these axons were not fully mature 9 months postgrafting, though they expressed axonal markers characteristic of both immature and mature axons such class III β-tubulin and light-chain neurofilament (NF70). Ultrastructural analysis revealed that all graft-host synapses had clear, spheroid vesicles presynaptically and displayed features characteristic of an excitatory phenotype. NF200 expression (not expressed by grafted neurons) in axons spanning across the graft demonstrated the regeneration of primate axons in a neural stem cell graft.
Monkeys were scored in a motor task that assessed >25 features of motor function including object manipulation and climbing. Monkeys with surviving grafts exhibited improved peak performance in motor function over time as compared to monkeys without surviving grafts (p = 0.02).
Image: PD
©2018 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.