Hydrocortisone does not improve bronchopulmonary dysplasia outcomes in pre-term babies
1. In extremely preterm infants on mechanical ventilation, hydrocortisone was unable to improve 36-week survival or 2-year survival without neurodevelopmental impairment compared to placebo.
Evidence Rating Level: 1 (Excellent)
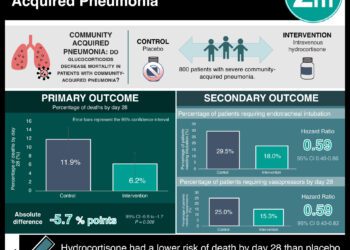
Study Rundown: Extremely preterm babies are at high risk for bronchopulmonary dysplasia (BPD), which is damage to the lungs caused by mechanical ventilation. Some evidence suggests that the administration of anti-inflammatory medications may reduce alveolar damage. To investigate this, infants born extremely preterm (< 30 weeks gestational age) who received mechanical ventilation were randomized to either receive hydrocortisone tapered over a period of 10 days or a placebo. Primary outcomes measures were efficacy (survival without BPD at 36 weeks postmenstrual age) and safety (survival without moderate or severe neurodevelopmental impairment at 2 years follow-up). Secondary endpoints assessed standard complications and growth measures following extremely pre-term delivery such as successful extubation and respiratory status. The study found that hydrocortisone did not improve the proportion of patients who survived without bronchopulmonary dysplasia at 36 weeks, nor the proportion of infants without neurodevelopmental impairment at 2 years compared to placebo. However, hydrocortisone was able to improve several secondary outcomes including an increased rate of successful extubation and shorter time on mechanical ventilation. With respect to adverse events, hydrocortisone treatment increased the frequency of hypertension. Taken together, the study does not support the use of hydrocortisone to improve BPD outcomes in extremely preterm infants. The results of this study are limited potential confounding by dexamethasone administration across both groups and its stringent inclusion criteria.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: In this large, multicenter, phase 3 randomized control trial, 800 infants born extremely preterm (< 30 weeks gestational age) and intubated for at least 7 days were enrolled. Infants were randomized in a 1:1 ratio to either receive oral or intravenous hydrocortisone (4 mg/kg bodyweight) tapered over 10 days or a placebo. The primary outcome of this study was divided into efficacy and safety measures. For efficacy, infants were assessed at 36 weeks for survival without moderate or severe bronchopulmonary dysplasia (BPD) defined by supplemental oxygen use, ventilation requirements, and an ambient-air challenge. For safety, survival at 22-26 months of corrected age without moderate or severe neurodevelopmental impairment was assessed according to Bayley Scales of Infant and Toddler Development-III (Bayley-III), Gross Motor Function Classification System (GMCFS), and vision/hearing examination. Secondary outcomes assessed standard progression from mechanical intubation such as rate and time to extubation, as well as severe adverse events. Most statistical differences were assessed by robust Poisson regression to obtain adjusted relative risk estimates. The study found that at 36 weeks of post-menstrual age, 16.6% of patients in the hydrocortisone group survived without moderate or severe BPD, which was not statistically different compared to 13.2% in the placebo group (adjusted rate ratio [ARR], 1.27; 95% confidence interval [CI], 0.93-1.74). Similarly, in the safety measure, 36.9% of infants receiving hydrocortisone survived without moderate or severe neurodevelopmental impairment, which was not statistically different from 37.3% in the placebo group (ARR, 0.98; 95% CI, 0.81-1.18). Hydrocortisone was able to improve a few secondary measures including an increased rate of successful extubation (44.7% vs. 33.6%) and fewer days of ventilation (mean, 37 days vs. 40 days). For severe adverse events, 4.3% of infants receiving hydrocortisone experienced hypertension, which was significantly greater than the placebo group (ARR, 4.27; 95% CI, 1.45-12.55). All other severe adverse events were similar between the two groups. Overall, the study does not support the use of hydrocortisone in extremely preterm infants on mechanical ventilation owing to its inability to improve efficacy and safety outcomes relating to BPD.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.