Hydroxychloroquine ineffective as post-exposure prophylaxis for Covid-19
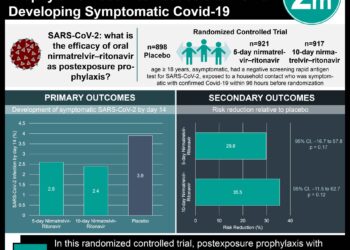
1. Hydroxychloroquine use as post-exposure prophylaxis within 4 days of a moderate or high-risk exposure did not prevent illness compatible with Covid-19 when compared to placebo.
2. Approximately 40% of individuals taking hydroxychloroquine reported side effects, all of which were noted to be mild and GI-related. There were no serious drug-related side effects observed.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The breadth of the morbidity and mortality worldwide from coronavirus disease 2019 (Covid-19) has led to an intensification of efforts to develop novel therapeutics aimed to reduce disease transmission and severity. Hydroxychloroquine has been closely studied within this context. This study employed a randomized, double blinded design to examine hydroxychloroquine use in post-exposure prophylaxis for Covid-19. Hydroxychloroquine use showed no significant reduction in the incidence of a Covid-19 illness in adults when taken within 4 days of a moderate or high-risk exposure. The study’s findings added to a growing body of literature which has yet to establish the efficacy of hydroxychloroquine within Covid-19. Limitations of this study included its reliance on a symptom driven Covid-19 identification as opposed to molecular testing and dependence on participants self-reporting all symptoms, medication adherence, and testing results. Lastly, its largely online based recruitment and survey distribution strategy likely pre-selected a healthier participant pool and may limit its generalizability. Of note, the study provided evidence for the relative safety of hydroxychloroquine. Side effects, while reported in over 40% of subjects taking the medication, were largely mild in nature and there were no reported arrythmias or deaths associated with its use. Such a finding is relevant in light of multiple recently retracted studies demonstrating potential harm associated with hydroxychloroquine use in Covid-19.
Clear here to read the study in the NEJM.
Relevant reading: Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19
In-Depth [randomized controlled trial]: The purpose of this study was to investigate if hydroxychloroquine used as postexposure prophylaxis could reduce symptomatic infections following covid-19 exposure. Asymptomatic adults were recruited, predominantly online, based on self-reported exposure to Covid-19 within 4 days. An exposure was defined as contact with a person with confirmed Covid-19 for at least 10 minutes either without a face mask or eye shield, termed a high risk exposure, or a face mask but no eye shield, termed as a moderate risk exposure. A total of 821 participants were enrolled, 66.4% of whom were healthcare workers. The hydroxychloroquine regimen was an initial dose of 800mg, followed by 600mg 6-8 hours later, and then 600mg daily for 4 days. The primary outcome was the development of a symptomatic illness via formal polymerase chain reaction (PCR) testing or the development of symptoms consistent with a Covid-like illness, after independent review of symptoms by four infectious disease specialists. The incidence of Covid-19 did not differ significantly between those receiving hydroxychloroquine (11.8%) or placebo (14.3%) (p =0.35) and the difference between groups was -2.4% (95% CI -7.0 to 2.2). Of participants taking hydroxychloroquine, 75.4% endorsed total compliance with the prescribed regimen and 40.1% reported a side effect at day 5, compared to 82.8% and 16.8%, respectively, in those taking the placebo. The most common symptoms reported from those taking hydroxychloroquine were nausea, loose stools, and abdominal discomfort. There were no reported serious drug-related reactions including arrythmias or death.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.