Infusion of CCR5-modified autologous CD4 T cells safe for HIV patients
Image: PD
1. Transfer of autologous CD4 T cells, with genome editing of the CCR5 gene, to HIV patients was successful and safe
2. Levels of modified CD4 T cells declined at a lower rate than unmodified CD4 T cells when antiretroviral therapy was interrupted
Evidence Rating Level: 2 (Good)
Study Rundown: The discovery of the human chemokine receptor 5 (CCR5), a protein on the surface of CD4 T cells, has led to substantial treatment advances in human immunodeficiency virus (HIV) infection. This transmembrane protein interacts with HIV envelope and is used by the virus to gain entry into cells. Individuals homozygous for a 32bp deletion (delta32/delta32) in the CCR5 gene were found to be immune to infection by the virus. Recent studies with molecular inhibitors that block CCR5 have been shown to be effective in humans. The purpose of this study was to investigate whether genomic editing of CD4 T cells at the CCR5 site is feasible and safe for HIV patients. Adenoviral vectors expressing a zinc-finger nuclease (ZFN) were used to knock out the CCR5 gene. The infusion was found to be well tolerated and safe overall. Transferred modified cells persisted after infusion with an average half-life of 48 weeks. When antiviral therapy was stopped, viremia recurred; the CCR5 knock out cells, however, were depleted at a much slower rate than unmodified cells. It should be noted that there is some flexibility in DNA recognition by ZFN, making off-targeting effects a concern. This study was also limited by its small sample size. Overall, this study was a significant step towards developing new therapies for HIV as well as advancing the field of genomic editing.
Click to read the study in NEJM
Click to read an accompanying editorial in NEJM
Relevant Reading: Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases
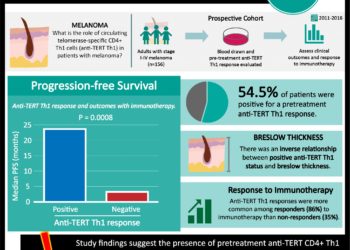
In-Depth [non-randomized control trial]: The study consisted of 12 patients divided in two case series. All patients had chronic aviremic HIV infections while they received highly active antiretroviral therapy (HAART). Patients were infused with autologous CD4-enriched T cells that were modified at the CCR5 gene locus by ZFNs. The primary objective of the study was to assess the safety and side-effect profile of the infusion. The secondary objective was to assess the effect of the infusion on CD4 count, persistence of the modified cells, homing of cells to gut mucosa, and effects on viral load. The infusion was well tolerated with the exception of one patient who developed a transfusion reaction. The median CD4 T-cell count was 517 per cubic millimeter at week 1, which was significantly increased from the count of 448 per cubic millimeter (P<0.001). The median concentration of CCR5-modified cells constituted 8.8% of circulating peripheral-blood mononuclear cells at week 1. Modified cells were found to have an estimated mean half-life of 48 weeks. Viremia recurred when treatment was interrupted. CCR5-modified cells decreased at a rate of -1.81 cells per day as compared to -7.25 cells per day of unmodified cells (P = 0.02).
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.