Key drugs reduce fracture risk in osteoporosis
1. This systematic review demonstrated that in postmenopausal women with osteoporosis, bisphosphonates, denosumab and teriparatide were associated with a significantly reduced risk of vertebral and nonvertebral fractures, compared to placebo.
2. The comparative effectiveness of the different pharmacological agents available to treat osteoporosis is unclear.
Evidence Rating Level: 1 (Excellent)
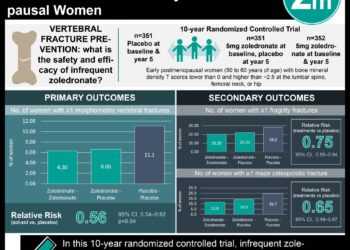
Study Rundown: Osteoporosis is a disease characterized by reduced bone mineral density (BMD) and increased fracture risk. There are multiple pharmacological agents available used for the treatment of osteoporosis. This study included 294 systematic reviews and randomized controlled trials (RCTs) pertaining to drugs approved by the U.S. Federal Drug Administration (FDA) for primary osteoporosis, except for calcitonin and etidronate. This systematic review found high-strength evidence that in postmenopausal women with osteoporosis, bisphosphonates, denosumab and teriparatide were associated with a reduced risk of vertebral and nonvertebral fractures compared to placebo. There was limited evidence identified on the comparative effectiveness of the different pharmacological agents available to treat osteoporosis, although the study did suggest that all-resorptives were significantly more effective than vitamin D and calcium supplements in preventing fractures. The evidence on adverse effects was generally weak and many findings were inconsistent. It was also noted that alendronate, teriparatide and denosumab were associated with mild GI disturbances. Furthermore, the results suggested that cases of osteonecrosis of the jaw were associated with intravenous bisphosphonates, atypical subtrochanteric fracture was associated with longer duration of bisphosphonate treatment, and denosumab was associated with increased infection risk. The limitations of these analyses relate to differences in the study types, inclusion criteria, outcomes and lengths of follow-up.
Click to read the study in Annals of Internal Medicine
Relevant Reading: Time Course of Bone Mineral Density Changes With Denosumab Compared With Other Drugs in Postmenopausal Osteoporosis: A Dose-response Based Meta-analysis
In-Depth [systematic review]: This study was a follow-up to an AHRQ-funded study from 2011. It was initiated because of new data showing that zoledronic acid prevented all fractures at 24 and 36 months (RR 0.23 to 0.73) and two RCTs on denosumab, the newly introduced agent, reported reduced risk of hip, vertebral, nonvertebral and new clinical vertebral fractures (HR 0.31 to 0.8). Medical literature database searches yielded 55,086 titles from which 294 systematic reviews or RCTs on FDA-approved drugs for primary osteoporosis, except for calcitonin and etidronate, were eligible for inclusion. Bisphosphonates (alendronate, ibandronate, risedronate, and zoledronic acid), denosumab and teriparatide, compared to placebo, reduced the risk of vertebral fracture (RR 0.40 to 0.60) and nonvertebral fracture (RR 0.60 to 0.80) in postmenopausal women with osteoporosis. The number needed to treat over 1 to 3 years to prevent 1 vertebral fracture was 60 to 89 people and 50 to 67 people to prevent 1 hip fracture. Furthermore, there was limited evidence directly comparing bisphosphonates, denosumab or teriparatide in fracture prevention.
More from this author: Rituximab not beneficial long-term in Sjorgen’s SyndromeHigh-energy shockwave therapy effective in treating shoulder calcific tendinitisTenofovir prophylaxis linked with reduced risk of herpes simplex 2
Image: PD
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.