Lacosamide may be safe and effective supplemental anti-epileptic drug
[tabs tab1=”2MM Rundown” tab2= “2MM Full Report”]
[tab]
Image: PD
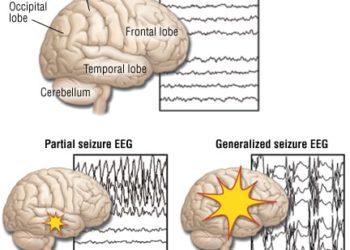
1. Lacosamide functions well as an add-on anti-epileptic drug (AED), decreasing frequency of seizures in 66% of patients with brain tumors.
2. Lacosamide is well tolerated with the majority (77%) of patient experiencing no side effects.
This retrospective study demonstrates the potential for lacosamide to be effective and safe as an add-on therapy for recurrent seizures in patients with brain tumors. The drug has many strengths as an AED for use specifically with brain tumor patients. Key attributes include lack of interactions with hepatic enzymes and low potential for drug interactions, useful for patients on extensive chemotherapy regimens. Given that this was a retrospective medical record review, it is subject to the typical limitations of a retrospective studies. However, the data presented does favor use of lacosamide as an AED in brain tumor patients, and a prospective study to clearly delineate the safety and efficacy of the drug is justified and warranted.
Click to read the study in JNS
Click to read an accompanying editorial in JNS
[/tab]
[tab]
Image: PD
1. Lacosamide functions well as an add-on anti-epileptic drug (AED), decreasing frequency of seizures in 66% of patients with brain tumors.
2. Lacosamide is well tolerated with the majority (77%) of patient experiencing no side effects.
This [retrospective, multicenter] study reviewed medical records from 5 US academic institutions, identifying over 70 patients with primary brain tumors who were taking lacosamide. A majority of these patients (74%) were taking lacosamide for recurrent seizures. 66% of patients reported decreased seizure frequency and 30% report stable seizures. 23% of patients required an additional AED to control their seizures. Of note, lacosamide was most effective in combination with levetiracetam compared to monotherapy (71% vs 33% decreased seizure frequency). The drug was well tolerated with 77% of patients reporting no toxicity. The most common side effect encountered was fatigue (6%), followed by dizziness, nausea, confusion, and weakness (2% each).
In sum: This retrospective study demonstrates the potential for lacosamide to be effective and safe as an add-on therapy for recurrent seizures in patients with brain tumors. The drug has many strengths as an AED for use specifically with brain tumor patients. Key attributes include lack of interactions with hepatic enzymes and low potential for drug interactions, useful for patients on extensive chemotherapy regimens. Given that this was a retrospective medical record review, it is subject to the typical limitations of a retrospective studies. However, the data presented does favor use of lacosamide as an AED in brain tumor patients, and a prospective study to clearly delineate the safety and efficacy of the drug is justified and warranted.
Click to read the study in JNS
Click to read an accompanying editorial in JNS
By Allen Ho and Marc Succi
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT. Content is produced in accordance with fair use copyrights solely and strictly for the purpose of teaching, news and criticism. No benefit, monetary or otherwise, is realized by any participants or the owner of this domain.
[/tab]
[/tabs]