Large population based study in Denmark predicts the rates of certain adverse outcomes temporally associated with vaccine administration [BMJ]
Image: CC/Sanofi Pasteur
Key study points:
1. There is marked variability in the incidence rates of the diseases linked with childhood vaccinations.
2. Occurrence rates of each disease followed a different distribution with regard to age, gender, and season.
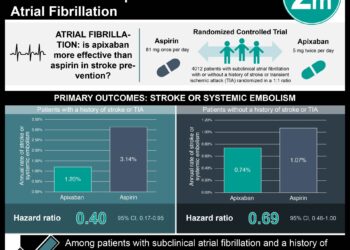
3. Of 1,000,000 vaccinations, approximately 7 adverse events are expected to occur within 1 day, 46 within 7 days, 276 within 42 days, and 1181 within 128 days after vaccine administration.
Primer: With the great advances that have been made in vaccine development and the continued growth of the field of preventative medicine, worries have arisen concerning the safety of immunizations. As it is often difficult to distinguish diseases that are temporally co-occurring from those that are causally linked to a vaccination event, questions regarding the risk of adverse outcomes have remained largely unanswered. The authors of this article studied rates of certain diseases (e.g. Guillain-Barre syndrome, acute transverse myelitis, optic neuritis, demyelinating disorders, seizures, anaphylaxis, Bell’s palsy, autoimmune thrombocytopenia, type 1 diabetes mellitus, juvenile and rheumatoid arthritis, narcolepsy, and death) in a large cohort of subjects in a population given standard childhood vaccinations. In doing so, they sought to develop background rates for these diseases, which may be then utilized to differentiate temporality from causality.
This [population based cohort] study: All infants born in Denmark between January 1, 1980 and December 31, 2009 were followed from birth until one of the following events occurred: diagnosis with one of the diseases of interest, emigration, age 18 years, endpoint of the study (December 31, 2009) or death. All subjects were provided standard immunizations through the Danish childhood vaccine program. The incidence of each diagnosis of interest was then utilized to constructed a “predicted event count” per 1,000,000 people vaccinated at various follow-up time points (1, 7, 42, and 182 days post immunization).
Of the 2,300,227 infants included in the study, 73,564 cases of the selected diagnoses were observed. There were considerable differences in the incidence rates of each disease, ranging from rare (autoimmune thrombocytopenia, at 0.32 per 100,000 person years) to relatively common (seizures, at 189.82 per 100,000 person years). Further, there was variability in disease occurrence with age and with season of the year. Finally, of 1,000,000 vaccinations, there would be an expected:
– 6.56 (10 = 80% upper bound, 14 = 99% upperbound) adverse events within 1 day
– 45.92 (53 = 80% upper bound, 63 = 99% upperbound) adverse events within 7 days
– 275.5 (290 = 80% upper bound, 316 = 99% upperbound) adverse events within 42 days
– 1180.7 (1211 = 80% upper bound, 1262 = 99% upperbound) adverse events within 128 days
In sum: This is the largest study to date providing incidence rates for the diseases/adverse outcomes that are most often cited in discussions of vaccine safety within the pediatric population. However, the authors note several major limitations. First, patients treated in the primary care, non-hospital setting were not included in this analysis and thus the calculated values may underestimate the actual incidences of the various diseases studied. Second, medical charts were not reviewed to validate the accuracy of the diagnoses. Third, the population studied may limit the applicability of this article’s findings to populations of different racial, ethnic, and environmental backgrounds. Finally, subsequent events occurring after the primary disease diagnosis were not accounted for, including patients who experienced recurrence of the primary event or the occurrence of another designated disease. Nevertheless, the authors argue that this publication provides “high quality estimates of the expected number of medical events that will be temporally associated with a vaccine dose during mass immunization,” thus providing health authorities with a key resource to discuss the risks and benefits of vaccines with a population worried about the safety of childhood immunization.
Written by M.K.
Click to read the study, published today in British Medical Journal
© 2012 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. DISCALIMER: Posts are not medical advice and are not intended as such. Please see a healthcare professional if you seek medical advice.