Lisinopril and carvedilol reduce cardiotoxicity in breast cancer patients receiving trastuzumab and anthracyclines
1. In this randomized, placebo controlled study of HER2-positive breast cancer patients receiving trastuzumab, use of preventative lisinopril or carvedilol did not significantly reduce cardiotoxicity compared to placebo.
2. For patients undergoing therapy with trastuzumab combined with anthracyclines, use of either carvedilol or lisinopril was associated with a reduced risk of cardiotoxicity compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: For patients with HER2-positive breast cancer, the use of trastuzumab has overcome a traditionally poor prognostic factor and leads to substantial improvements in overall survival and cancer-related morbidity. Trastuzumab has well established cardiotoxicity and can lead to reduction in left ventricular function and clinical heart failure. This can be compounded when trastuzumab is combined with anthracyclines. This study was conducted to evaluate the effectiveness of lisinopril and carvedilol in reducing the development of cardiotoxicity in HER2-positive breast cancer patients receiving trastuzumab with or without anthracycline chemotherapy. The study found that, compared to placebo, use of lisinopril or carvedilol did not reduce risk of cardiotoxicity with the use of trastuzumab. Use of preventative carvedilol and lisinopril were demonstrated to have reduced risk of cardiac toxicity when the patients were receiving trastuzumab and anthracyclines.
The main strength of the study was the randomized, double blind, placebo controlled design allowing for minimization of bias and accurate estimation of risk reduction. The limitations of the study included the non-standardized methodology for estimating left ventricular (LV) function, and the lack of power to compare carvedilol to lisinopril to establish which agent may be preferred.
Click to read the study in JACC
Relevant Reading: Trastuzumab-induced cardiotoxicity and its risk factors in real-world setting of breast cancer patients
In-Depth [randomized controlled trial]: This study was a double-blind, multicenter, randomized, placebo-controlled trial evaluating lisinopril and carvedilol for prevention of cardiotoxicity in patients with early-stage HER2-positive breast cancer scheduled to receive 12 months of trastuzumab therapy. Patients were stratified according to whether they were also receiving anthracyclines as part of their therapy. Patients with baseline reduction of LV function, or major cardiovascular risk factors were excluded from the trial. The primary outcome was the rate of a cardiotoxicity event defined as a decrease in LV ejection fraction (LVEF) of ≥10% in patients whose LVEF was ≥50%, or a drop in LVEF of at least 5% from baseline in patients whose LVEF decreases to <50%.
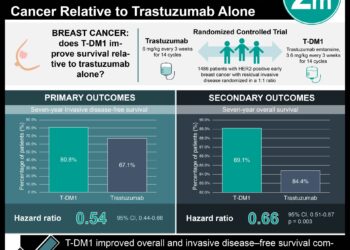
A total of 468 women were included in the study, with 189 having received combination of trastuzumab and anthracycline therapy. There was no significant difference in cardiotoxicity between lisinopril (30%), carvedilol (29%), or placebo (32%) overall. For the trastuzumab-alone cohort, there was no difference in outcomes with either carvedilol (HR 1.05; 95% CI 0.57 – 1.93) or lisinopril (HR 1.17; 95% CI 0.62 – 2.2) compared to placebo. However, for the cohort of patients who received both trastuzumab and anthracycline therapy, both carvedilol (HR 0.49; 95% CI 0.27 – 0.89) and lisinopril (HR 0.53; 95% CI 0.30 – 0.94) reduced cardiotoxicity-free survival compared with placebo. There was not enough data to directly compare carvedilol to lisinopril.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.