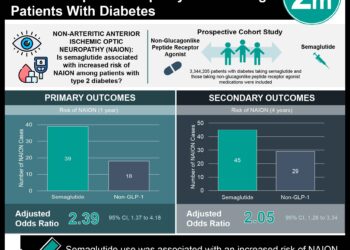
Long-term mortality benefit of BP lowering in type 2 diabetes [ADVANCE-ON trial]
1. The mortality benefit of blood pressure (BP) lowering therapy observed in the ADVANCE trial persisted at 6-year post-trial follow-up.
2. Intensive glucose control showed no benefit in reducing overall mortality or major macrovascular events compared to standard glucose control.
Evidence Rating Level: 2 (Good)
Study Rundown: The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial was a multicentre, randomized controlled study that included over 10,000 patients with type 2 diabetes. The 2-by-2 factorial trial assessed the benefit of BP-lowering therapy compared to placebo, and intensive glucose control compared to standard glucose control to important clinical outcomes in this group. One of the main findings was that BP-lowering with combined perindopril and indapamide was associated with a reduction in overall mortality and death from cardiovascular causes. Results of the trial also showed that intensive glucose control, defined as a glycated hemoglobin level less than 6.5%, did not in-crease mortality and reduced the incidence of combined major macrovascular and microvascular events, primarily due to a reduction in the incidence of nephropathy.
The ADVANCE observational study (ADVANCE-ON) is a post-trial follow-up of surviving partici-pants from the ADVANCE trial for a median of 5.9 years. Main findings from the post-trial follow-up include that the mortality benefit of BP-lowering therapy remained significant though attenuat-ed at six years post-trial and there was no long-term benefit of intensive glucose control on overall mortality or major macrovascular events when compared to standard glucose control. This study contributes to our understanding of long-term clinical outcomes of therapy in patients with type 2 diabetes. Limitations include incomplete follow-up of the original trial participants and less stringent post-trial follow-up procedures (often by telephone or questionnaires).
Click to read the study in NEJM
In-Depth [observational study]: This post-trial follow-up observational study included 8,494 pa-tients of 11,140 participants from the original trial. The primary outcomes measured were all-cause mortality and major macrovascular events (composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular cause). The median in-trial and post-trial follow-up periods were 4.4 years and 5.9 years, respectively, for the BP-lowering comparison and 5.0 years and 5.4 years, respectively, for the glucose control comparison.
During the randomized ADVANCE trial, there was a significant between-group difference in blood pressure between the group receiving combined perindopril-indapamide and the placebo group. This difference was no longer evident 6 months after the trial and blood pressure levels were similar between the two groups during the post-trial period. The reduction in all-cause mortality observed in the perindopril-indapamide group during the trial remained significant but attenuated in the post-trial follow-up (hazard ratio, 0.91; 95% CI, 0.84 to 0.99; P=0.03). The significant be-tween-group difference in glycated haemoglobin levels observed in the randomized trial was no longer evident at the first post-trial visit and levels remained similar throughout the post-trial peri-od. There was no benefit observed with intensive glucose control on all-cause mortality or major macrovascular events compared to standard glucose control.
Image: PD
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without ex-pressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.