Long-term prophylactic co-trimoxazole beneficial for HIV-positive children in Africa
Image: PD
1. Among HIV-positive African children being treated with anti-retroviral therapy, continuing rather than stopping prophylactic co-trimoxazole led to less morbidity and mortality.
Evidence Rating Level: 1 (Excellent)
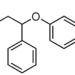
Study Rundown: Antiretroviral therapy (ART) directly antagonizes the human immunodeficiency virus and is therefore the cornerstone of treatment for HIV-positive individuals. However, since HIV wreaks its damage by hobbling the body’s ability to defend itself from infection, prophylactic antibiotics can be an important supplemental therapy. Co-trimoxazole (trimethoprim-sulfamethoxazole) is an inexpensive, broad-spectrum antibiotic that has been shown to reduce morbidity and mortality in certain HIV-positive populations. In African populations, co-trimoxazole prevents opportunistic infections, such as Pneumocystis jirovecii, as well as malarial and non-opportunistic bacterial infections. In sub-Saharan Africa, co-trimoxazole is often given to HIV-positive children before and during ART. However, it has been unclear when or if co-trimoxazole should be stopped. This study found significant benefits to continuing co-trimoxazole, even after 2 years of ART and even if the immune system appears healthy. The high rate of serious bacterial and protozoal infections in sub-Saharan Africa makes co-trimoxazole prophylaxis useful and relevant. It may not be as useful in a setting with lower rates of such infections.
Click to read the study in NEJM
Relevant Reading: Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1 infected adults in Abidjan, Côte d’Ivoire: a randomized trial
In-Depth [randomized controlled trial]: Almost 800 children aged 3 or older in Uganda and Zimbabwe who had been receiving ART for more than 96 weeks were randomized to either stop or continue daily co-trimoxazole and were followed for more than 120 weeks. This was an open-label trial and those stopping co-trimoxazole were not given placebo pills. The primary end points were hospitalization or death. There was a greater risk of a primary end point occurring among those who stopped co-trimoxazole compared to those who continued (19% vs. 13%; HR 1.64; p=0.007). There was also a greater risk of malaria among those who stopped the antibiotic (20% vs. 10%; HR 2.21; p<0.001). In terms of safety, there was no significant difference in the rate of adverse events between the stopped- and continued-prophylaxis groups (17% vs. 15%; p =0.33). However, there was a higher rate of severe adverse events among the stopped-prophylaxis group (3% vs. 1%; p= 0.04). Overall, the study shows convincingly that among children in sub-Saharan Africa, prolonged co-trimoxazole prophylaxis reduces morbidity and mortality. It also appears to be relatively safe.
By Tomi Jun and Xu Gao
More from this author: Early risk factor for progression of cystic fibrosis identified, Gut microbes implicated in stroke and heart attacks: new dietary link, New leukemia mutation offers therapeutic targets, Childhood ADHD associated with increased risk of suicide, A marker of aggressive liver cancer and potential therapeutic target identified
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.