Low risk of nephrogenic systemic fibrosis with group II gadolinium contrast agents in chronic kidney disease
1. In a systematic review and meta-analysis of the use of group II (low risk) gadolinium-based contrast agents (GBCA) for MRI in patients with stage 4 and 5 chronic kidney disease (CKD), there were no reported cases of nephrogenic systemic fibrosis (NSF).
2. The use of gadobenate dimeglumine, which is partially eliminated through hepatic metabolism, had the greatest number of reported patients and therefore the best estimated safety profile.
Evidence Rating Level: 2 (Good)
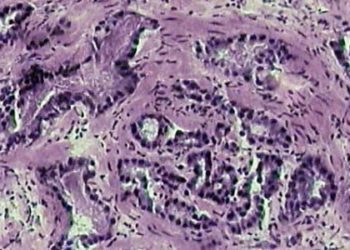
Study Rundown: Nephrogenic systemic fibrosis is a rare complication of gadolinium exposure and risk is linked to decreased gadolinium renal clearance. Original guidelines recommended against exposure to GBCA if the creatinine clearance is less than 30 ml/min/1.73m2. However, the use of newer formulations of (group II) GBCA has reduced the risk of NSF and the avoidance of contrast in patients with renal insufficiency may lead to indirect harms of delayed or missed diagnosis. The current study is a systematic review and meta-analysis which evaluated the incidence and risk of NSF in patients with stage 4 and 5 CKD receiving group II GBCA. The study found that in a pooled sample of 4931 patients there was no reported cases of NSF and the upper 95% confidence interval limit for incidence was 0.07%. Gadobenate dimeglumine, an agent with some hepatic clearance, had the largest number of patients studied and therefore the greatest safety margin.
The main strengths of the meta-analysis include the large sample size, and low estimated bias in most domains. The main limitations of the study include the reliance on predominantly retrospective data with unblinded assessments, and the lack of universal standard for the diagnosis of NSF.
Click to read the study in JAMA Internal Medicine
Relevant Reading: A Systematic Review of 639 Patients with Biopsy-confirmed Nephrogenic Systemic Fibrosis
In-Depth [systematic review and meta-analysis]: This study is a systematic review and meta-analysis which included studies that evaluated patients with stage 4 or 5 CKD who underwent administration of a group II GBCA (gadobenate dimeglumine, gadobutrol, gadoterate meglumine, or gadoteridol) and reported incidence of NSF. Conference abstracts, retracted manuscripts, case reports, and studies not reporting total group II GBCA administrations were excluded.
The meta-analysis included 16 studies comprised of 4931 total patients. There were no reported cases of NSF with incidence of 0% (upper bound of 95%CI, 0.07%). The most common GBCA was gadobenate dimeglumine with 3167 patients (upper bound of 95%CI, 0.12%).
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.