Magnesium for the prevention of cerebral palsy [Classics Series]
This study summary is an excerpt from the book 2 Minute Medicine’s The Classics in Medicine: Summaries of the Landmark Trials
1. Among women with imminent preterm delivery, babies of women randomized to magnesium were less likely to be diagnosed with moderate or severe cerebral palsy.
Original Date of Publication: August 2008
Study Rundown: Cerebral palsy, the leading cause of childhood disability, is a group of syndromes characterized by motor and postural dysfunction. A strong risk factor for cerebral palsy is preterm birth, which is associated with as many as a third of cerebral palsy cases. In the early-mid 2000s, research identified an increased prevalence of cerebral palsy among very preterm infants (28-32 weeks gestation). The survival of preterm (< 37 weeks gestation) and very preterm infants born in the United States drastically improved in recent decades such that more premature infants survive to childhood. In the setting of improved survival, researchers have been working to prevent, mitigate and treat morbidities associated with prematurity, including cerebral palsy. A retrospective investigation identified a lower odds ratio of cerebral palsy among premature infants whose mothers received magnesium. However, previous studies enrolled women in preterm labor, who are often recommended to receive magnesium for its purposes as a uterine tocolytic. The present study achieves greater parsimony by enrolling women without other indications for magnesium therapy to specifically evaluate the benefit of intrapartum magnesium for fetal neuroprotection in premature infants born between 24 and 31 weeks.
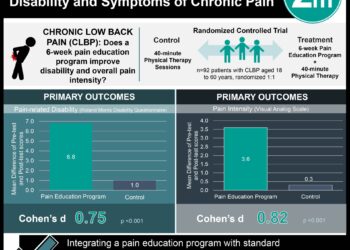
This randomized, placebo-controlled trial evaluated the benefit of intrapartum administration of magnesium sulfate for fetal neuroprotection in women at risk for imminent preterm delivery between 24 and 31 weeks gestation. Follow-up exams at 2 years of age showed a decrease of almost 50% in the diagnosis of moderate or severe cerebral palsy in infants whose mothers received magnesium. Along with other studies, this landmark investigation indicated that magnesium could significantly reduce the risk of cerebral palsy in the group of infants at highest risk for developing this disabling condition. Strengths included elegant study design and high follow-up rate of 96%. Inclusion of women with both singleton and twin gestations receiving care at 20 delivery centers across the United States allows for widely applicable results. Limitations included potential selection bias, with 40% of eligible participants (1602/3843) declining to participate.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: Multicenter trial of 2241 women at risk for imminent delivery between 24 and 31 weeks of gestation were randomly assigned to receive magnesium sulfate (6 g loading dose followed by 2 g/hour infusion) or placebo. Women with hypertension, pre-eclampsia, or those requiring magnesium for tocolysis were excluded. Outcomes assessed included the primary composite outcome of stillbirth or infant death within 1 year. Secondary outcomes assessed were moderate or severe cerebral palsy beyond 2 years of age. The diagnosis of cerebral palsy was rigorously determined by an annually certified pediatrician or pediatric neurologist. Diagnosis was made if children met 2 or more strictly defined criteria, including motor delay, abnormal muscle tone or reflexes, persistence of primitive reflexes, and movement abnormality.
Among women with imminent delivery between 24 and 31 weeks gestation, those randomized to receive magnesium sulfate were 45% less likely to be diagnosed with moderate or severe cerebral palsy at 2 years of life (1.9% vs. 3.5%, RR 0.55; p = 0.03). Risk of fetal death did not differ between groups (9.5 vs. 8.5%, p = 0.4). Obstetrical outcomes and a range of other neonatal outcomes, including respiratory distress, intraventricular hemorrhage, necrotizing enterocolitis and retinopathy of prematurity were similar between groups (all p > 0.5).
Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. The New England Journal of Medicine. 2008;359(9):895-905.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc