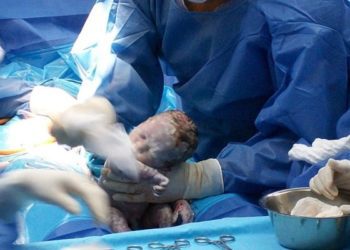
Maternally administered vaccine reduces infant group B streptococcus infection
1. In this randomized controlled trial, a maternally administered vaccine for infant group B streptococcus (GBS) demonstrated a reduced risk of invasive GBS.
2. There was no significant difference in number of adverse events seen in patients who received the vaccine.
Evidence Rating Level: 1 (Excellent)
Study Rundown: GBS can cause sepsis and meningitis in newborns, with the primary risk factor being exposure to maternal rectovaginal group B streptococcal colonization during delivery. Often, pregnant women receive intrapartum antibiotic prophylaxis if they test positive for the GBS screen. Recently, a hexavalent CPS-cross-reactive material 197 glycoconjugate vaccine (GBS6) was developed as a maternal vaccine to prevent invasive GBS in infants. However, there is a gap in knowledge as to understanding the effectiveness of GBS6 and whether anti-CPS antibodies are transferred to newborn infants. Overall, this study found that GBS6 elicited anti-CPS antibodies against GBS in pregnant women, and transferred these antibodies to the infants at levels that are indicative of reduced risk for invasive GBS in newborn infants. This study was limited by not studying concurrent infections during pregnancy such as malaria or HIV, which can affect placental integrity and antibody transfer. Nevertheless, these study’s findings are significant, as they demonstrate that the GBS6 maternal vaccine for GBS may reduce the risk of infants developing invasive GBS disease.
Click here to read the study in NEJM
Relevant Reading: Azithromycin to Prevent Sepsis or Death in Women Planning a Vaginal Birth
In-Depth [randomized controlled trial]: This phase two placebo-controlled trial was conducted at three research centers in South Africa. Patients who were healthy pregnant women 18 to 40 years of age were eligible for the trial. Patients who did not provide informed consent were excluded from the trial. Eligible patients then received a single dose of 5 μg, 10 μg, or 20 μg per serotype of GBS6 with or without aluminum phosphate (AlPO4) or placebo. The primary outcome measured were anti-CPS IgG concentrations, which were determined using a quantitative direct immunoassay that measured antibody levels binding to CPS serotypes Ia, Ib, and II through V. Outcomes in the primary analysis were assessed via the Clopper and Pearson method with point estimates and exact 2-sided 95% confidence intervals. Based on the primary analysis, GBS6 induced effective maternal antibody responses, with maternal-to-infant antibody ratios of approximately 0.4 to 1.3. The percentage of infants with anti-CPS IgG concentrations above 0.184 μg per milliliter was 57 to 97% depending on vaccine formulation, which is the threshold determined to be associated with a 75% reduction in risk of disease. Overall, this study demonstrates that the GBS6 vaccine elicits anti-CPS antibodies against GBS in pregnant women that also subsequently transfer to infants at levels that would significantly reduce the risk of invasive GBS in the infants.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.