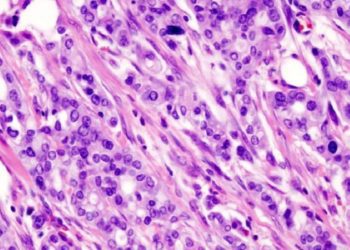
Nanoliposome allows for targeted drug delivery to treat pancreatic cancer [PreClinical]
1. The authors synthesized photoactivable multi-inhibitor nanoliposomes (PMILs), lipid nanoparticles that encapsulated the chemotherapeutic cabozantinib and released drug in response to light.
2. In mouse models of pancreatic ductal adenocarcinoma (PDAC), PMIL treatment led to a significant decrease in tumor size, microvascular proliferation, and metastasis.
Evidence Rating Level: 2 (Good)
Study Rundown: The MET protein can confer cancer cell resistance to antiangiogenic treatment, which prevents blood vessel growth. Although the MET inhibitor cabozantinib is a highly effective potential cancer therapy, due to its high toxicity, an improved delivery method is needed to target the drug specifically to cancer cells. To this end, the authors encapsulated the drug within a lipid bilayer containing a photoactivable chromophore. The chromophore allowed for controlled release of the drug from the PMIL upon exposure to near-infrared irradiation. The PMIL was tested in two mouse models of PDAC, in which human PDAC cells were grafted either subcutaneously or within the pancreas. Compared to cabozantinib, empty liposome, and both administered simultaneously, PMIL treatment significantly decreased tumor size in both models. In the second model, PMIL treatment also significantly decreased microvascular proliferation as well as liver and lung metastases. Although this treatment was toxic to the tumors, it produced no significant systemic toxicity in the mice.
This study demonstrated the potential of the PMIL as a safer way to administer an otherwise toxic drug. Notably, the two components of the PMIL have already shown clinical efficacy; cabozantinib is used to treat thyroid cancer, while the light-activated chromophore is used to treat age-related macular degeneration. Since there is currently no highly effective treatment for tumor growth or metastasis in pancreatic cancer, this work shows promise in addressing the unmet need in the field.
Click to read the study in Nature Nanotechnology
Relevant Reading: Effects of integrin-targeted photodynamic therapy on pancreatic carcinoma cell
In-Depth [animal study]: When constructing the liposome, benzoporphyrin derivative, a photoactivable chromophore, was incorporated into the lipid bilayer. Nanoparticles containing cabozantinib were then incorporated into the liposome. After optimizing the construction of the nanoliposome, confocal microscopy was used to determine its internalization efficiency. The PMIL was successfully internalized by a PDAC cell line. Next, in vitro efficacy of the PMIL in targeting PDAC cells was assessed. Western blotting showed that although MET activation was inhibited upon photorelease of cabozantinib from the PMIL, there were no changes in the total levels of the protein. Compared to treatment with empty liposome or empty liposome and cabozantinib together, PMIL treatment caused increased cancer cell death.
To evaluate the PMIL in vivo, human PDAC cells were xenografted into mice underneath the skin or in the pancreas. PMIL treatment was administered intravenously 18 days after subcuteneous implantation and 10 days after pancreatic implantation, and tumors were irradiated 1 hour after treatment administration. PMIL not only led to a 92% reduction in subcutaneous tumor size, but this effect was sustained over 10 days. This reduction in size was significantly more than the ~50% tumor reduction caused by treatment with the empty liposome alone, drug alone, and simultaneous empty liposome and drug administration (p<0.001). Similar results were obtained with pancreatic implantation of the cancer cells. Not only was tumor size significantly decreased, but microvascular volume within the tumor was also significantly decreased (p<0.01). Finally, metastatic burden was assessed using a qRT-PCR assay on the liver and iliac lymph nodes of the mice. After 6 days of treatment, there was a 98.7% reduction of metastasis compared to no treatment.
Image: CC/Wiki/Andrea Trementozzi
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.