Nedaplatin-based concurrent chemoradiotherapy is noninferior to cisplatin-based therapy in patients with locoregional nasopharyngeal carcinoma
1. Patients receiving either nedaplatin-based or cisplatin-based concurrent chemoradiotherapy had no significant difference in progression free survival at five years.
2. There were no significant differences in rates of overall survival, distant metastasis-free survival or locoregional relapse between the nedaplatin and cisplatin groups.
Level of Evidence: 1 (Excellent)
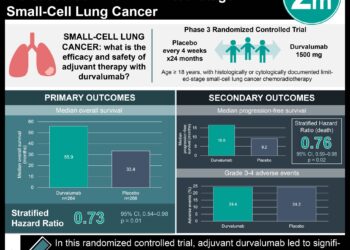
Study Rundown: Nasopharyngeal cancer is a common and highly comorbid malignancy in parts of South and East Asia. The current standard of management for locoregional (Stages II to IVb) disease consists of cisplatin-based concurrent chemoradiotherapy (CCRT) administered every three weeks. Early evidence suggests that nedaplatin, an analogue of cisplatin, has similar efficacy to cisplatin and may have fewer associated adverse events. The present study is a secondary analysis of a randomized controlled trial comparing nedaplatin and cisplatin-based CCRT in patients with locoregional nasopharyngeal cancer. 402 patients were randomized in this study (201 cisplatin, 201 nedaplatin). The 5-year survival rate was 81.4% in the cisplatin group and 79.8% in the nedaplatin group in the intention-to-treat analysis. The overall survival was also not statistically significant between groups, at 89.4% in the cisplatin group and 88.8% in the nedaplatin group. Finally, the cumulative incidence of distant metastasis was 26.9% in the cisplatin group and 22.8% in the nedaplatin group; this difference was also not statistically significant. Toxicity was assessed amongst a subgroup of 398 patients. Cisplatin was associated with an increased risk of high-grade auditory toxic events than nedaplatin; no other significant differences in adverse event rates were noted. The secondary analysis of this trial demonstrated that nedaplatin chemoradiotherapy was nonsignificant to cisplatin in the management of locoregional nasopharyngeal cancer, and that the risk of auditory toxic events may be reduced. Some strengths of this study include randomization which helps to control for confounding bias, as well as the thoroughness of the analysis over a lengthy follow-up period. A primary limitation of the study was that patients in the nedaplatin group were less likely to complete the trial than the cisplatin group; this could have introduced bias to the analysis of adverse event rates. It is also difficult to determine whether adverse events experienced were due to the radiation therapy (standard across both arms) or the chemotherapeutic agent.
Click to read this study in JAMA
Relevant Reading: Concurrent chemoradiotherapy for loco-regionally advanced nasopharyngeal carcinoma: treatment outcomes and prognostic factors
In-Depth [randomized, controlled trial]: All patients received standard intensity-modulated radiotherapy as a part of their CCRT regime. Nedaplatin was delivered as a 2-hour infusion and cisplatin as a 4-hour infusion every 3 weeks for 3 cycles. The completion rate for all three cycles was 97.5% in the nedaplatin group and 57.2% in the cisplatin group. The eligibility criteria to enroll in this study were as follows: patients aged 18-65 with Stage T1-4N-3 or T3-4N0 nasopharyngeal cancer, normal hematological/renal/hepatic parameters and Karnofsky performance status of at least 70. Blocked randomization was performed manually and stratified by treatment center and disease stage. The 5-year survival rate was 81.4% (95% confidence interval [CI] 75.9-86.9%) in the cisplatin group and 79.8% [95% CI, 74.1%-85.5%] in the nedaplatin group; the absolute difference in rates was 1.6%, which was below the non-inferiority margin of 10%. The hazard ratio for overall survival in the cisplatin versus nedaplatin group was 1.15 (95% CI 0.66-2.01), indicating nonsignificance. The hazard ratio for the rate of distant metastases in the cisplatin versus nedaplatin groups was 0.75 (95% CI 0.44-1.28). The rate of Grade 3 and 4 auditory toxic events was 10.4% in the nedaplatin group and 17.7% in the cisplatin group (p = 0.04). The odds ratio for auditory toxicity in the nedaplatin group versus the cisplatin group was 0.51 (95% CI 0.28-0.93, p =0.03). The rates of the following adverse events were not different between treatment arms: trismus, dysphagia, skin/soft tissue symptoms, dry mouth, peripheral neuropathy, cranial neuropathy, endocrine dysfunction and temporal lobe necrosis. The findings of noninferiority was robust to sensitivity analyses with the following covariates: sex, age (younger or older than 45 years), Karnofsky performance status, and disease stage.
Imaged: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.