New migraine-specific pharmacologic agents associated with less efficacy compared to triptans
1. For pain freedom and relief at 2 hours after dosage, new agents such as lasmiditan, rimegepant, and ubrogepant were associated with a higher odds ratio compared to placebo, but a lower odds ratio compared to most triptans.
2. The lack of cardiovascular risks among new classes of migraine-specific pharmacologic agents may provide alternative treatment options for patients with migraine attacks and cardiovascular contraindications to triptans.
Evidence Rating Level: 1 (Excellent)
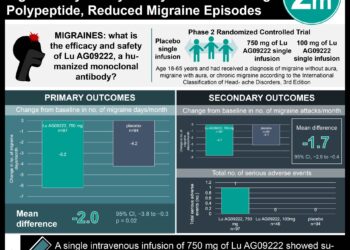
Study Rundown: The current standard of care for the treatment of acute migraine centers around triptans, a class of selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonists. More recently, new therapeutic classes of migraine-specific pharmacologic agents have been developed, including 5-hydroxytryptamine1F (5-HT1F) receptor agonists (lasmiditan) and calcitonin gene-related peptide (CGRP) antagonists (rimegepant and ubrogepant). This systematic review and network meta-analysis compared the efficacy of newer agents including 5-HT1F receptor agonists and CGRP antagonists compared to triptans for the treatment of acute migraine attacks. The main endpoint was the odds ratio (OR) for freedom from pain at 2 hours after dosage, where secondary outcomes included ORs for pain relief at 2 hours and any adverse events. Among 64 randomized clinical trials (RCTs) with a total of 46,442 patients, triptans, ditans, and gepants were associated with reduced pain at 2 hours compared to placebo. However, most triptans were associated with reduced pain when compared with newer agents (ditans and gepants), where ditans had the highest risk of adverse events among all treatments and certain triptans carried a higher risk of adverse events compared to gepants. For pain freedom and pain relief at 2 hours after dosage, lasmiditan, rimegepant, and ubrogepant were associated with a higher OR compared to placebo, but a lower OR compared to most triptans. However, the lack of cardiovascular risks among these new classes of migraine-specific pharmacologic agents may provide alternative treatment options for patients with migraine attacks and cardiovascular contraindications to triptans. A limitation of this study was the sole focus on short-term responses and adverse events after a single dose of pharmacologic treatment, where long-term safety and efficacy of gepants and ditans need to be investigated in future clinical studies.
Click to read the study in JAMA Network Open
Relevant Reading: Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial
In-Depth [systematic review and meta-analysis]: This systematic review and network meta-analysis included a total of 64 double-blind RCTs examining current migraine-specific acute treatments (46,442 participants; 74%-87% women; age range, 36-43 years) searched from inception to March 2020 among databases including the Cochrane Register of Controlled Trials, Embase, and PubMed. Data extraction was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline and all network meta-analyses were conducted using a random effects model. Overall, most new pharmacologic agents were associated with reduced pain at 2 hours compared to placebo. However, for pain freedom at 2 hours, triptans were associated with higher ORs compared to new agents including lasmiditan (range: OR, 1.72 [95%CI, 1.06-2.80] to OR, 3.40 [95%CI, 2.12-5.44]), rimegepant (range: OR, 1.58 [95%CI, 1.07-2.33] to OR, 3.13 [95%CI, 2.16-4.52]), and ubrogepant (range: OR, 1.54 [95%CI, 1.00-2.37] to OR, 3.05 [95%CI, 2.02-4.60]). Similarly, for pain relief at 2 hours, triptans were associated with higher ORs vs lasmiditan (range: OR, 1.46 [95%CI, 1.09-1.96] to OR, 3.31 [95%CI, 2.41-4.55]), rimegepant (range: OR, 1.33 [95%CI, 1.01-1.76] to OR, 3.01 [95%CI, 2.33-3.88]), and ubrogepant (range: OR, 1.38 [95%CI, 1.02-1.88] to OR, 3.13 [95%CI, 2.35-4.15]). Among new treatments, comparisons between lasmiditan, rimegepant, and ubrogepant did not yield statistically significant results for both pain freedom and pain relief at 2 hours, where lasmiditan was also found to have the highest risk of any adverse events.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.