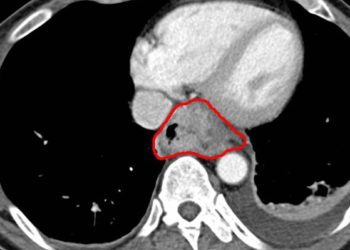
Nivolumab plus ipilimumab increases overall survival in patients with malignant pleural mesothelioma
1. Median overall survival in the nivolumab plus ipilimumab group (18.1 months) was substantially greater than in the chemotherapy group (14.1 months).
2. Rate of grade 3-4 treatment-related adverse events was similar between groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Malignant pleural mesothelioma (MPM) is a highly aggressive cancer with a poor five-year prognosis. In recent years, platinum agents and folate antimetabolites have been used as the first-line treatment for MPM. However, long-term survival outcomes remain poor with chemotherapy. Nivolumab plus ipilimumab has shown clinical benefit in other tumor types, such as non-small-cell lung cancer, but have yet to be tested for MPM. This multicenter, randomized controlled trial aimed to compare the efficacy and safety of first line nivolumab plus ipilimumab with platinum plus pemetrexed chemotherapy in unresectable MPM. The primary outcome for this study was overall survival in patients. Secondary outcomes included progression-free survival, response rate, time to response, duration of response, and disease control rate. Results showed a significant increase in medial overall survival in the nivolumab plus ipilimumab group compared to chemotherapy . In addition, the likelihood of high-grade treatment-related adverse events were similar with nivolumab plus ipilimumab and pemetrexed chemotherapy. While the interim endpoints were met after approximately two and a half years of follow-up, five and ten-year follow up data would be interesting to evaluate in future studies. Nevertheless, this study is the first to show a significant improvement in overall survival with immunotherapy versus platinum plus chemotherapy for treatment of MPM.
Click to read the study in The Lancet
Relevant Reading: Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma – Phase 1b Results From the JAVELIN Solid Tumor Trial
In-depth [randomized controlled trial]: Between Nov 29, 2016, and Apr 28, 2018, 713 patients were enrolled in the study at 103 hospitals across 21 countries. Included patients were those aged 18 years or older with histologically confirmed unresectable MPM and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients with brain metastases, autoimmune disease, and previous treatment with drugs targeting T-cell costimulation or checkpoint pathways were excluded. Altogether, 605 patients were randomly assigned (303 in nivolumab plus ipilimumab and 302 in chemotherapy) and included in the analysis. Baseline characteristics were similar across both groups. The median age among patients was 69 years (interquartile range [IQR] 64-75) and 77% (467 of 605) were male.
After a median follow-up of 29.7 months (IQR 26.7-32.9), median overall survival was found to be significantly improved with nivolumab plus ipilimumab (18.1 months, 95% confidence interval [CI] 16.8-21.4) compared to chemotherapy (14.1 months, 95% CI 12.4-16.2; hazard ratio [HR] 0.74, p=0.0020). At 1-year follow-up, overall survival rates were higher in the nivolumab plus ipilimumab group (68%, 95% CI 62.3-72.8) than the chemotherapy group (58%, 95% CI 51.7-63.2). A similar pattern was observed at 2 years from baseline with nivolumab plus ipilimumab (41%, 95% CI 35.1-46.5) versus chemotherapy (27%, 95% CI 21.9-32.4). In contrast, the secondary outcome of median progression-free survival showed marginal differences between treatment groups: 6.8 months (95% CI 5.6-7.4) with nivolumab plus ipilimumab and 7.2 months (95% CI 6.9-8.0) with chemotherapy (HR 1.00, 95% CI 0.82-1.21). This was also true for disease control with a median time to response of 2.5 months (IQR 1.45-3.27) for nivolumab plus ipilimumab and 2.5 months (IQR 1.41-3.02) for chemotherapy. Although the frequency of grade 3-4 treatment-related adverse events was similar in both groups (30% vs. 32%), fewer deaths were reported with nivolumab plus ipilimumab (n=198, 66%) than with chemotherapy (n=212, 75%). Findings from this study suggest that nivolumab plus ipilimumab has a favorable benefit-risk profile and should be considered for treatment of unresectable MPM.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.