Oral azacytidine maintenance therapy improved survival outcomes in patients with acute myeloid leukemia
1. Oral azacytidine also known as CC-486 has been found to improve survival outcomes in patients with acute myeloid leukemia.
2. Frequently reported adverse events associated with oral azacytidine maintenance therapy were nausea, vomiting and diarrhea. However, the frequency of these events reduced with ongoing treatment cycles as a possible result of dose adjustment and medication initiation.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Acute myeloid leukemia (AML) is predominantly present in the elderly population although treated with chemotherapy, often results in relapse and reduced survival. This randomized, double-blind, placebo-control trial was conducted to assess overall survival and adverse effects of CC-486 as a maintenance therapy in patients with AML. Participants were recruited and randomized into one of two groups. The first group received oral azacytidine (CC-486) and the second group received a placebo. Participants were monitored until death, or if consent was withdrawn, or if loss to follow-up occurred. The most common adverse events as a result of CC-486 or placebo administration were nausea, vomiting and diarrhea. The study showed a significant difference and longer relapse-free survival period in the treatment group when compared to the placebo group. The study was strengthened by its large sample size and can be generalized to the larger patient population.
Click to read the study in NEJM
Relevant Reading: Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia
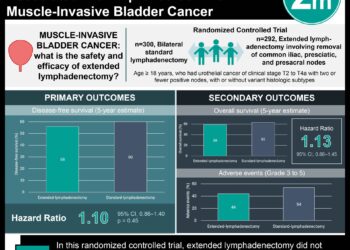
In-Depth [randomized controlled trial]: This phase 3 trial was conducted across 23 countries at 148 sites from May 2013 to October 2017 with a data cutoff date of July 2019. Patient eligibility was determined using the following: over 55 years of age, new diagnosis of acute myeloid leukemia or a secondary diagnosis of AML or a poor-risk associated with the diagnosis of AML and have received chemotherapy and in remission at time of screening. A total of 472 patients were enrolled and randomized into the treatment group where they received 300 mg of CC-486 or into the placebo group. A total of 245 were male and 227 were female participants of which the median age was 68 years. The treatment was administered once a day for fourteen days and the cycle was repeated every 28 days. Ongoing adverse events were noted throughout the follow up period and laboratory assessments were conducted for the first 24 cycles for every 3 cycles and the dosing regimen was modified based on the results. The calculated power of the study was 90% based on a total enrollment of 460 patients. The study had a dropout rate of 5% and a 60-month average trial duration. The placebo group overall survival time was 14.8 months compared to the CC-486 group for which it was 24.7 months and the median duration of the treatment was 6 cycles versus 12 cycles respectively. Both groups demonstrated similar adverse events (i.e.; gastrointestinal including nausea, vomiting and diarrhea) as a result of treatment. Neutropenia and thrombocytopenia were also reported as a result of treatment in both groups. In conclusion, both groups demonstrated similar adverse events, however there was a longer relapse-free survival period as a result of oral azacytidine maintenance therapy.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article