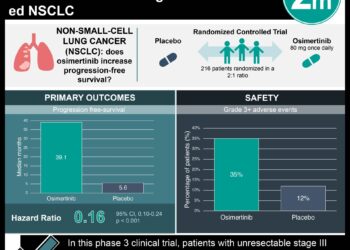
Osimertinib significantly improved progression-free survival in patients with unresectable stage III non-small cell lung cancer
1. Treatment with osimertinib significantly improved progression-free survival, compared to placebo, in patients with unresectable stage III EGFR-mutated NSCLC
2. The safety profile of osimertinib was consistent with previous studies, with most adverse effects being grade 1 or 2, and manageable
Evidence Rating Level: 1 (Excellent)
Study Rundown: In this phase 3, double-blind, placebo-controlled trial, 216 patients with unresectable stage III EGFR-mutated non-small cell lung cancer (NSCLC) who had received chemoradiotherapy, were then randomly assigned to receive osimertinib (143 patients) or placebo (73 patients). The primary endpoint was progression-free survival (PFS). Key secondary endpoints included overall survival, survival without progression of CNS disease, objective response rate, duration of response, and safety. This study suggests that treatment with osimertinib significantly improves PFS when compared to placebo in patients with stage III EGFR-mutated NSCLC. Although adverse events of grade 3 or higher were more common in the osimertinib group, the safety profile of osimertinib was felt to be consistent with previous studies, with most adverse events being grade 1 or 2. Limitations of this study include the immature overall survival data and crossover design. The study also included a primarily Asian population which may limit generalizability to non-Asian populations. Overall, the results of this phase 3 LAURA trial suggest that osimertinib could become the new standard of care for this patient population.
Click to read the study in NEJM
Relevant Reading: Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC
In-Depth [placebo-controlled trial]: In this trial, 216 patients, over the age of 18, with locally advanced, unresectable stage III NSCLC with an EGFR mutation and a WHO PSS of 0 or 1 were assigned in a 2:1 ratio to receive either oral osimertinib at a dose of 80 mg per day or placebo until disease progression. Baseline patient characteristics in both groups were similar. The progression-free survival in the osimertinib group was 39.1 months (95% CI, 31.5 to not calculable), compared to only 5.6 months (95% CI, 3.7 to 7.4) in the placebo group; overall HR for disease progression or death was 0.16 (95% CI, 0.10 to 0.24; P<0.001). Patients in the osimertinib group were found to have lower incidences of local progression (21% compared to 48% in the placebo group), lower incidences of distant metastases (16% compared to 37% in the placebo group), and lower incidences of new lesions (22% compared to 68% in the placebo group). The median duration of response was 36.9 months with osimertinib, compared to only 6.5 months with placebo. Adverse events were reported in 98% of patients receiving osimertinib and 88% of patients in the placebo group. The most common adverse events were radiation pneumonitis, diarrhea, and rash. Adverse events of grade 3 or higher were reported in 35% of the patients receiving osimertinib, compared to only 9% of those receiving placebo. Limitations of this study include the fact that the overall survival data were not mature at the time of reporting, making it difficult to conclude the long-term survival benefits of osimertinib. Furthermore, a considerable proportion of patients in the placebo group (81%) crossed over to receive osimertinib after disease progression which may confound results. Another limitation stems from the fact that 81% of patients in the osimertinib arm and 85% of patients in the placebo arm were Asian; generalizability of findings to non-Asian populations may be difficult.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.