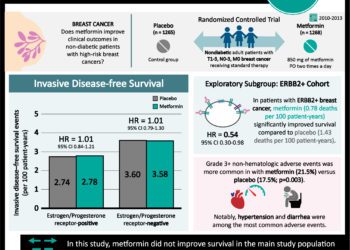
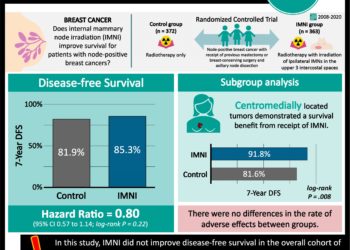
Palbociclib taken with letrozole increases progression-free survival in breast cancer patients
1. Patients with previously untreated estrogen receptor ER positive (ER+), human epidermal growth factor receptor 2 (HER2) negative breast cancer had longer progression-free survival times when taking palbociclib and letrozole compared to patients taking letrozole alone.
2. Serious adverse events were more common for patients taking palbociclib and letrozole compared to those taking letrozole.
Evidence Rating: 1 (Excellent)
Study Rundown: ER-positive breast cancers traditionally treated with hormone blockage can develop resistance to hormone blocking medications. Because of this, newer drugs have been developed for ER-positive breast cancer that don’t rely on hormonal effect. Cell cycle inhibitors of cyclin dependent kinases (CDKs) have shown the potential to inhibit ER-positive tumor growth. Initial studies of one such CDK inhibitor, palbociclib, have shown to be useful in combination with hormonal blocking drugs. This study sought to further assess differences in efficacy of a standard treatment with or without palbociclib, and better evaluate adverse outcomes of palbociclib when used in this regimen.
Postmenopausal ER+/HER2- breast cancer women were recruited into this study, and those who had significant disease related complications were excluded. One group was treated with palbociclib and letrozole while the other group was treated with letrozole and a placebo. Those in the palbociclib-letrozole group had a significantly longer median length of progression-free survival compared to the letrozole-placebo group. Serious adverse events related to treatment were more common in the palbociclib-letrozole group compared to the letrozole-placebo group. Many of the serious adverse events in the palbociclib-letrozole group were hematological (neutropenia, leukopenia, anemia).
Click to read the study, published today in NEJM
Relevant Reading: Targeting breast cancer with CDK inhibitors
In-Depth [randomized controlled trial]: This phase 3 study enrolled patients from 2013 to 2014 at 186 sites in 17 countries, and analysis was carried through until February 2016. Patients were assigned in a 2:1 ratio to palbociclib-letrozole (n = 444) and letrozole-placebo (n = 222) groups. Eligible women for the study were ER+, HER2 negative, postmenopausal, and had not received prior systemic therapy. Patients at risk for short-term, life-threatening complications due to their disease status were not included in the study. Palbociclib and placebo were administered in 1 month cycles of 125 mg orally daily for 3 weeks, and 1 week off. Letrozole was given as 2.5 mg/day orally. Patients were imaged regularly to assess for disease progression. Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events. Median progression-free survival for palbociclib-letrozole was 24.8 months while that of letrozole-placebo was 14.5 months, and median follow-up as 23 months (HR for disease progression or death, 0.58; 95%CI 0.46 to 0.72; two-sided p < 0.001). When patients were stratified according to disease characteristics (such as visceral disease, nonvisceral disease, and receiving or not receiving hormonal therapy previously) patients treated with palbociclib-letrozole all had significantly longer progression-free median survival times compared to letrozole-placebo. Patients in the palbociclib-letrozole group experienced serious adverse events, most often of a hematological nature (neutropenia, leukopenia, anemia), more frequently than those in the letrozole-placebo group (19.6% vs 12.6%). Statistical assessment of differences in adverse events between the two groups was not performed. Patients who experienced adverse events were mangaged with supportive care and reduction of their palbociclib dose.
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.



![Engineered stem cells mitigate liver damage caused by radiation [PreClinical]](https://www.2minutemedicine.com/wp-content/uploads/2014/12/Human_embryonic_stem_cells-75x75.png)



