Pharmacist-led extended medication review and follow-up on discharge may decrease risk of readmission
1. In this randomized clinical trial, a pharmacist-led extended intervention, which included medication review, three motivational interviews, and telephone follow-up resulted in significant decreases in readmission at 180 days.
2. The number needed to treat with the extended intervention as compared to usual care was 12. No cost-effective analysis was performed at this time.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Polypharmacy in our patients may put them at risk for readmission due to adverse drug reactions or drug-related issues. It is thought up to half of these readmissions may be preventable. This randomized controlled trial aimed to determine whether a multifaceted pharmacist intervention based on medication review, patient interview and follow-up could reduce readmissions and emergency department visits.
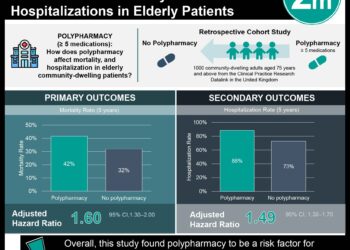
An extended intervention, which included medication review, three motivational interviews, and telephone follow-up resulted in significant decreases in readmission at 30 and 180 days. There was also a significant decrease in readmission or ED visit within 180 days with the extended intervention. The number needed to treat to achieve the primary composite outcome for extended intervention as compared to usual care was 12. Limitations of the study included its unblinded design. Next steps for study could include feasibility and cost-effective analysis.
Click to read the study, published in JAMA Internal Medicine
Relevant Reading: Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis
In-Depth [randomized trial]: This randomized, multi-center trial (Odense Pharmacist Trial Investigating Medication Interventions at Sector Transfer [OPTIMIST]) took place from September 2013 to October 2015 in 4 different acute admission wards in Denmark and included 1467 participants. Participants included in the study were 18 years or older and used 5 or more medications who were medical patients with an acute admission to the above Denmark medical wards. Exclusion criteria included: patients were declared terminally ill, suicidal, in custody, were in isolation precautions or had severe dementia or aphasia. Patients were randomized 1:1:1 to usual care, basic intervention (structured patient-centered medication review) and extended intervention group (medication review, medication reconciliation, 3 motivational interviews, with phone call follow-up interview). The outcome of interest was readmission within 30 or 180 days and composite outcome of readmission or emergency department visits within 180 days. Secondary outcomes were drug-related readmissions within 30 and 180 days after inclusion and all-cause mortality and drug-related mortality. Intention-to-treat analysis was used.
A total of 1467 patients were included in the primary analysis: 498 randomized to usual care, 493 to basic intervention and 476 to extended intervention. There was a significant decrease in readmission rates in the extended intervention group: within 30 days HR 0.62 (95% CI 0.46-0.84) and within 180 days HR 0.75 (95% CI 0.62-0.90). There was also a significant decrease in the primary composite outcome (HR 0.77, 95% CI 0.64-0.93). There were non-significant reductions in drug-related admissions. In all outcomes, the difference between basic interventions and usual care were non-significant. The number needed to treat to achieve the primary composite outcome for extended intervention as compared to usual care was 12.
Image: CC/Wiki
©2018 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.