Policy statement outlines recommendations on management of infants born to mothers with COVID-19
1. The American Academy of Pediatrics (AAP) recommends that newborns born to mothers affected by or being tested for COVID-19 be temporarily separated from their mothers. It is also recommended that these infants be separated from newborns born to unaffected mothers in order to reduce risk of transmission.
2. The report also recommends that for infants requiring evaluation at sites with available testing, molecular assays should be completed at 24 hours of life and then repeated at 48 hours. The AAP statement also includes recommendations for feeding with breast milk obtained by pumping adherent to cleaning protocols.
Statement Rundown: As the novel coronavirus SARS-CoV-2 and the disease it causes, COVID-19, continue to spread globally, limited data have been available regarding its transmission between pregnant women and newborns. The American Academy of Pediatrics (AAP) has published a report offering interim guidance for infants born to mothers with confirmed or suspected COVID-19. Key recommendations of this statement are listed below:
- Thus far, studies and case reports have documented COVID-19 in children as young as 2 days of age, and while pediatric cases are generally less severe than cases in older individuals, a number of infants have been reported to have severe or critical illness.
- Current evidence is insufficient to understand the potential for vertical transmission. The AAP recommends that neonates born to women with or being tested for COVID-19 should be considered as persons under investigation for the virus as well, and that proper droplet and contact precautions should be used for encounters. In addition, airborne precautions should be observed in situations with potential for the generation of aerosols. It is acknowledged that certain centers’ shortages of personal protective equipment may limit if individuals can follow this recommendation.
- Mothers and newborns should be separated to minimize the risk of postnatal infant infection. The likely benefits of separation should be discussed with mothers prior to delivery.
- Infants born to mothers with or being tested for COVID-19 should ideally be admitted to specific areas separate from areas for newborns unaffected by maternal COVID-19. Should an infant require neonatal intensive care, they should optimally be admitted to a single patient room with negative room pressure capabilities. Separation in the hospital should be maintained until the mother has been a) afebrile for 72 hours without the use of antipyretics, and b) at least 7 days have passed since symptoms first appeared.
- No study to date has demonstrated the presence of SARS-CoV-2 in breast milk, so breast milk expression and feeding should continue with appropriate cleaning of breast pumps and components.
- Should a mother choose to room-in with her infant, the infant should remain at least 6 feet from the mother at all times. If the mother requests skin-to-skin contact, she should wear a mask and ensure breast hygiene.
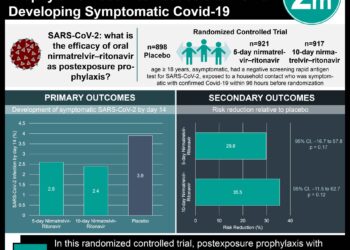
- Newborns born to COVID-19-affected mothers should be tested at 24 and 48 hours of age. If two tests obtained at least 24 hours apart are negative, the hospital may transition the infant to universal precautions.
- Infants testing positive but not displaying symptoms may be discharged home on a case-by-case basis with appropriate precautions and plans for follow-up, ideally not to individuals >60 years of age or those with underlying health conditions. Infants testing negative can be discharged home to the care of a healthy caregiver.
Click to read the policy statement, published in Pediatrics
Click to read the accompanying FAQ
Relevant reading: Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.